A high throughput microelectroporation device to introduce a chimeric antigen receptor to redirect the specificity of human T cells
- PMID: 20574820
- PMCID: PMC4109048
- DOI: 10.1007/s10544-010-9440-3
A high throughput microelectroporation device to introduce a chimeric antigen receptor to redirect the specificity of human T cells
Abstract
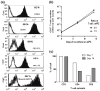
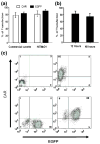
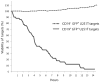
It has been demonstrated that a chimeric antigen receptor (CAR) can directly recognize the CD19 molecule expressed on the cell surface of B-cell malignancies independent of major histocompatibility complex (MHC). Although T-cell therapy of tumors using CD19-specific CAR is promising, this approach relies on using expression vectors that stably integrate the CAR into T-cell chromosomes. To circumvent the potential genotoxicity that may occur from expressing integrating transgenes, we have expressed the CD19-specific CAR transgene from mRNA using a high throughput microelectroporation device. This research was accomplished using a microelectroporator to achieve efficient and high throughput non-viral gene transfer of in vitro transcribed CAR mRNA into human T cells that had been numerically expanded ex vivo. Electro-transfer of mRNA avoids the potential genotoxicity associated with vector and transgene integration and the high throughput capacity overcomes the expected transient CAR expression, as repeated rounds of electroporation can replace T cells that have lost transgene expression. We fabricated and tested a high throughput microelectroporator that can electroporate a stream of 2 x 10(8) primary T cells within 10 min. After electroporation, up to 80% of the passaged T cells expressed the CD19-specific CAR. Video time-lapse microscopy (VTLM) demonstrated the redirected effector function of the genetically manipulated T cells to specifically lyse CD19+ tumor cells. Our biomedical microdevice, in which T cells are transiently and safely modified to be tumor-specific and then can be re-infused, offers a method for redirecting T-cell specificity, that has implications for the development of adoptive immunotherapy.
Figures




Similar articles
-
piggyBac transposon/transposase system to generate CD19-specific T cells for the treatment of B-lineage malignancies.Hum Gene Ther. 2010 Apr;21(4):427-37. doi: 10.1089/hum.2009.114. Hum Gene Ther. 2010. PMID: 19905893 Free PMC article.
-
Universal artificial antigen presenting cells to selectively propagate T cells expressing chimeric antigen receptor independent of specificity.J Immunother. 2014 May;37(4):204-13. doi: 10.1097/CJI.0000000000000032. J Immunother. 2014. PMID: 24714354 Free PMC article.
-
Treatment of advanced leukemia in mice with mRNA engineered T cells.Hum Gene Ther. 2011 Dec;22(12):1575-86. doi: 10.1089/hum.2011.070. Epub 2011 Sep 23. Hum Gene Ther. 2011. PMID: 21838572 Free PMC article.
-
Nonviral RNA transfection to transiently modify T cells with chimeric antigen receptors for adoptive therapy.Methods Mol Biol. 2013;969:187-201. doi: 10.1007/978-1-62703-260-5_12. Methods Mol Biol. 2013. PMID: 23296935 Review.
-
Allogeneic chimeric antigen receptor-modified cells for adoptive cell therapy of cancer.Expert Opin Biol Ther. 2014 Jul;14(7):947-54. doi: 10.1517/14712598.2014.900540. Epub 2014 Mar 24. Expert Opin Biol Ther. 2014. PMID: 24661086 Review.
Cited by
-
Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor.Blood. 2010 Aug 19;116(7):1035-44. doi: 10.1182/blood-2010-01-043737. Epub 2010 May 3. Blood. 2010. PMID: 20439624 Free PMC article. Review.
-
Gene therapy for cancer: present status and future perspective.Mol Cell Ther. 2014 Sep 10;2:27. doi: 10.1186/2052-8426-2-27. eCollection 2014. Mol Cell Ther. 2014. PMID: 26056594 Free PMC article. Review.
-
Chimeric antibody receptors (CARs): driving T-cell specificity to enhance anti-tumor immunity.Front Biosci (Schol Ed). 2012 Jan 1;4(2):520-31. doi: 10.2741/282. Front Biosci (Schol Ed). 2012. PMID: 22202074 Free PMC article. Review.
-
Microfluidic electroporation for cellular analysis and delivery.Lab Chip. 2013 Oct 7;13(19):3803-21. doi: 10.1039/c3lc50566a. Lab Chip. 2013. PMID: 23917998 Free PMC article. Review.
-
CARs in chronic lymphocytic leukemia -- ready to drive.Curr Hematol Malig Rep. 2013 Mar;8(1):60-70. doi: 10.1007/s11899-012-0145-y. Curr Hematol Malig Rep. 2013. PMID: 23225251 Free PMC article. Review.
References
-
- Baum C, Dullmann J, Li Z, Fehse B, Meyer J, Williams DA. Blood. 2003;101:2099. - PubMed
-
- Cooper LJN. Blood. 2008;112:2172. - PubMed
-
- Cooper LJN, Topp MS, Serrano LM, Gonzalez S, Chang W, Naranjo A. Blood. 2003;101:1637. - PubMed
-
- Cooper LJN, Al-Kadhimi Z, Serrano LM, Pfeiffer T, Olivares S, Castro A. Blood. 2005;105:1622. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

