Mib-Jag1-Notch signalling regulates patterning and structural roles of the notochord by controlling cell-fate decisions
- PMID: 20573700
- PMCID: PMC2927672
- DOI: 10.1242/dev.051011
Mib-Jag1-Notch signalling regulates patterning and structural roles of the notochord by controlling cell-fate decisions
Abstract
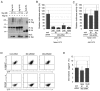
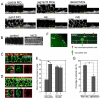
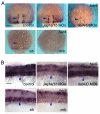
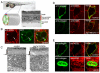
In the developing embryo, cell-cell signalling is necessary for tissue patterning and structural organization. During midline development, the notochord plays roles in the patterning of its surrounding tissues while forming the axial structure; however, how these patterning and structural roles are coordinated remains elusive. Here, we identify a mechanism by which Notch signalling regulates the patterning activities and structural integrity of the notochord. We found that Mind bomb (Mib) ubiquitylates Jagged 1 (Jag1) and is essential in the signal-emitting cells for Jag1 to activate Notch signalling. In zebrafish, loss- and gain-of-function analyses showed that Mib-Jag1-Notch signalling favours the development of non-vacuolated cells at the expense of vacuolated cells in the notochord. This leads to changes in the peri-notochordal basement membrane formation and patterning surrounding the muscle pioneer cells. These data reveal a previously unrecognized mechanism regulating the patterning and structural roles of the notochord by Mib-Jag1-Notch signalling-mediated cell-fate determination.
Figures


Similar articles
-
The ubiquitin ligase Drosophila Mind bomb promotes Notch signaling by regulating the localization and activity of Serrate and Delta.Development. 2005 May;132(10):2319-32. doi: 10.1242/dev.01825. Epub 2005 Apr 13. Development. 2005. PMID: 15829515
-
Jagged 1 regulates the restriction of Sox2 expression in the developing chicken inner ear: a mechanism for sensory organ specification.Development. 2011 Feb;138(4):735-44. doi: 10.1242/dev.060657. Development. 2011. PMID: 21266409
-
Jagged-Notch signaling ensures dorsal skeletal identity in the vertebrate face.Development. 2010 Jun;137(11):1843-52. doi: 10.1242/dev.049056. Epub 2010 Apr 28. Development. 2010. PMID: 20431122 Free PMC article.
-
Cell and molecular biology of Notch.J Endocrinol. 2007 Sep;194(3):459-74. doi: 10.1677/JOE-07-0242. J Endocrinol. 2007. PMID: 17761886 Review.
-
Anti-Jagged-1 immunotherapy in cancer.Adv Med Sci. 2022 Sep;67(2):196-202. doi: 10.1016/j.advms.2022.04.001. Epub 2022 Apr 11. Adv Med Sci. 2022. PMID: 35421813 Review.
Cited by
-
The role of ubiquitylation in nerve cell development.Nat Rev Neurosci. 2011 May;12(5):251-68. doi: 10.1038/nrn3009. Nat Rev Neurosci. 2011. PMID: 21505515 Review.
-
Spine Patterning Is Guided by Segmentation of the Notochord Sheath.Cell Rep. 2018 Feb 20;22(8):2026-2038. doi: 10.1016/j.celrep.2018.01.084. Cell Rep. 2018. PMID: 29466731 Free PMC article.
-
New classes of mind bomb-interacting proteins identified from yeast two-hybrid screens.PLoS One. 2014 Apr 8;9(4):e93394. doi: 10.1371/journal.pone.0093394. eCollection 2014. PLoS One. 2014. PMID: 24714733 Free PMC article.
-
Zebrafish enpp1 mutants exhibit pathological mineralization, mimicking features of generalized arterial calcification of infancy (GACI) and pseudoxanthoma elasticum (PXE).Dis Model Mech. 2014 Jul;7(7):811-22. doi: 10.1242/dmm.015693. Epub 2014 Jun 6. Dis Model Mech. 2014. PMID: 24906371 Free PMC article.
-
SNX17 regulates Notch pathway and pancreas development through the retromer-dependent recycling of Jag1.Cell Regen. 2012 Jun 28;1(1):4. doi: 10.1186/2045-9769-1-4. eCollection 2012. Cell Regen. 2012. PMID: 25408867 Free PMC article.
References
-
- Appel B., Fritz A., Westerfield M., Grunwald D. J., Eisen J. S., Riley B. B. (1999). Delta-mediated specification of midline cell fates in zebrafish embryos. Curr. Biol. 9, 247-247 - PubMed
-
- Artavanis-Tsakonas S., Rand M. D., Lake R. J. (1999). Notch signaling: cell fate control and signal integration in development. Science 284, 770-770 - PubMed
-
- Broder Y. C., Katz S., Aronheim A. (1998). The ras recruitment system, a novel approach to the study of protein-protein interactions. Curr. Biol. 8, 1121-1121 - PubMed
-
- Coutinho P., Parsons M. J., Thomas K. A., Hirst E. M., Saude L., Campos I., Williams P. H., Stemple D. L. (2004). Differential requirements for COPI transport during vertebrate early development. Dev. Cell 7, 547-547 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

