The effect of allosteric modulators on the kinetics of agonist-G protein-coupled receptor interactions in single living cells
- PMID: 20571079
- PMCID: PMC2939483
- DOI: 10.1124/mol.110.064493
The effect of allosteric modulators on the kinetics of agonist-G protein-coupled receptor interactions in single living cells
Abstract
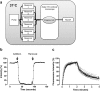
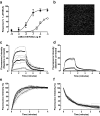
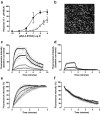
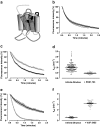
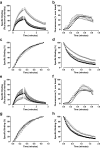
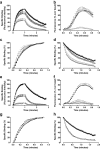
Allosteric binding sites on adenosine -A(1) and -A(3) receptors represent attractive therapeutic targets for amplifying, in a spatially and temporally selective manner, the tissue protective actions of endogenous adenosine. This study has directly quantified the kinetics of agonist/G protein-coupled receptor interactions at the single-cell level, reflecting the physiological situation in which intracellular signaling proteins can exert major allosteric effects on agonist-receptor interactions. The association and dissociation rate constants at both A(1) and A(3) receptors, and therefore the affinity of the fluorescent adenosine derivative ABA-X-BY630 (structure appears in J Med Chem 50:782-793, 2007), were concentration-independent. The equilibrium dissociation constants of ABA-X-BY630 at A(1) and A(3) receptors were approximately 50 and 10 nM, respectively, suggesting that, even in live cells, low agonist concentrations predominantly detect high-affinity receptor states. At A(1) receptors, the dissociation of ABA-X-BY630 (30 nM) was significantly faster in the absence (k(off) = 1.95 +/- 0.09 min(-1)) compared with the presence of the allosteric enhancer (2-amino-4,5-dimethyl-3-thienyl)(3-(trifluoromethyl)phenyl)-methanone (PD81,723; 10 microM; k(off) = 0.80 +/- 0.03 min(-1)) and allosteric inhibitor 4-methoxy-N-(7-methyl-3-(2-pyridinyl)-1-isoquinolinyl)benzamide (VUF5455; 1 microM; k(off) = 1.48 +/- 0.16 min(-1)). In contrast, ABA-X-BY630 dissociation from A(3) receptors was significantly slower in the absence (k(off) = 0.78 +/- 0.18 min(-1)) than in the presence of the allosteric inhibitors VUF5455 (1 microM; k(off) = 3.15 +/- 0.12 min(-1)) and PD81,723 (10 microM; k(off) = 2.46 +/- 0.18 min(-1)). An allosteric mechanism of action has previously not been identified for PD81,723 at the A(3) receptor or VUF5455 at the A(1) receptor. Furthermore, the marked enhancement in fluorescent agonist dissociation by VUF5455 in living cells contrasts previous observations from broken cell preparations and emphasizes the need to study the allosteric regulation of agonist binding in living cells.
Figures

Similar articles
-
Allosteric enhancers of A1 adenosine receptors increase receptor-G protein coupling and counteract Guanine nucleotide effects on agonist binding.Mol Pharmacol. 2003 Dec;64(6):1557-64. doi: 10.1124/mol.64.6.1557. Mol Pharmacol. 2003. PMID: 14645687
-
Allosteric modulation of A(3) adenosine receptors by a series of 3-(2-pyridinyl)isoquinoline derivatives.Mol Pharmacol. 2001 Nov;60(5):1057-63. Mol Pharmacol. 2001. PMID: 11641434 Free PMC article.
-
The allosteric enhancer PD81,723 increases chimaeric A1/A2A adenosine receptor coupling with Gs.Biochem J. 2006 May 15;396(1):139-46. doi: 10.1042/BJ20051422. Biochem J. 2006. PMID: 16390330 Free PMC article.
-
Allosteric modulators for adenosine receptors: an alternative to the orthosteric ligands.Curr Top Med Chem. 2010;10(10):976-92. doi: 10.2174/156802610791293136. Curr Top Med Chem. 2010. PMID: 20370657 Review.
-
Allosteric Modulators of the Class A G Protein Coupled Receptors.Adv Exp Med Biol. 2016;917:185-207. doi: 10.1007/978-3-319-32805-8_9. Adv Exp Med Biol. 2016. PMID: 27236557 Review.
Cited by
-
The use of fluorescence correlation spectroscopy to characterize the molecular mobility of fluorescently labelled G protein-coupled receptors.Biochem Soc Trans. 2016 Apr 15;44(2):624-9. doi: 10.1042/BST20150285. Biochem Soc Trans. 2016. PMID: 27068980 Free PMC article. Review.
-
Allosteric modulation of purine and pyrimidine receptors.Adv Pharmacol. 2011;61:187-220. doi: 10.1016/B978-0-12-385526-8.00007-2. Adv Pharmacol. 2011. PMID: 21586360 Free PMC article. Review.
-
Use of a new proximity assay (NanoBRET) to investigate the ligand-binding characteristics of three fluorescent ligands to the human β1-adrenoceptor expressed in HEK-293 cells.Pharmacol Res Perspect. 2016 Aug 8;4(5):e00250. doi: 10.1002/prp2.250. eCollection 2016 Oct. Pharmacol Res Perspect. 2016. PMID: 27588207 Free PMC article.
-
Allosteric interactions at adenosine A(1) and A(3) receptors: new insights into the role of small molecules and receptor dimerization.Br J Pharmacol. 2014 Mar;171(5):1102-13. doi: 10.1111/bph.12345. Br J Pharmacol. 2014. PMID: 24024783 Free PMC article. Review.
-
Lighting up G protein-coupled purinergic receptors with engineered fluorescent ligands.Neuropharmacology. 2015 Nov;98:58-67. doi: 10.1016/j.neuropharm.2015.04.001. Epub 2015 Apr 16. Neuropharmacology. 2015. PMID: 25890205 Free PMC article. Review.
References
-
- Avlani V, May LT, Sexton PM, Christopoulos A. (2004) Application of a kinetic model to the apparently complex behavior of negative and positive allosteric modulators of muscarinic acetylcholine receptors. J Pharmacol Exp Ther 308:1062–1072 - PubMed
-
- Birdsall NJ, Lazareno S, Matsui H. (1996) Allosteric regulation of muscarinic receptors. Prog Brain Res 109:147–151 - PubMed
-
- Bruns RF, Fergus JH. (1990) Allosteric enhancement of adenosine A1 receptor binding and function by 2-amino-3-benzoylthiophenes. Mol Pharmacol 38:939–949 - PubMed
-
- Christopoulos A, Kenakin T. (2002) G protein-coupled receptor allosterism and complexing. Pharmacol Rev 54:323–374 - PubMed
-
- Christopoulos A, Lanzafame A, Ziegler A, Mitchelson F. (1997) Kinetic studies of co-operativity at atrial muscarinic M2 receptors with an “infinite dilution” procedure. Biochem Pharmacol 53:795–800 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
