Induction of anti-glioma natural killer cell response following multiple low-dose intracerebral CpG therapy
- PMID: 20570924
- PMCID: PMC3022005
- DOI: 10.1158/1078-0432.CCR-09-3087
Induction of anti-glioma natural killer cell response following multiple low-dose intracerebral CpG therapy
Abstract
Purpose: Stimulation of toll-like receptor-9 by CpG oligodeoxynucleotides (CpG-ODN) has been shown to counteract the immunosuppressive microenvironment and to inhibit tumor growth in glioma models. These studies, however, have used high doses of CpG-ODN, which can induce toxicity in a clinical setting. The goal of this study was to evaluate the antitumor efficacy of multiple low-dose intratumoral CpG-ODN in a glioma model.
Experimental design: Mice bearing 4-day-old intracranial GL261 gliomas received a single or multiple (two or four) intratumoral injections of CpG-ODN (3 microg) every 4 days. Tumor growth was measured by bioluminescent imaging, brain histology, and animal survival. Flow cytometry and cytotoxicity assays were used to assess anti-glioma immune response.
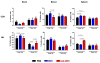
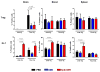
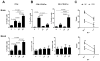
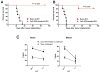
Results: Two and four intracranial injections of low-dose CpG-ODN, but not a single injection, eradicated gliomas in 70% of mice. Moreover, surviving animals exhibited durable tumor-free remission (> 3 months) and were protected from intracranial rechallenge with GL261 gliomas, showing the capacity for long-term antitumor immunity. Although most inflammatory cells seemed to increase, activated natural killer (NK) cells (i.e., NK(+)CD107a(+)) were more frequent than CD8(+)CD107a(+) in the brains of rechallenged CpG-ODN-treated animals and showed a stronger in vitro cytotoxicity against GL261 target cells. Leukocyte depletion studies confirmed that NK cells played an important role in the initial CpG-ODN antitumor response, but both CD8 and NK cells were equally important in long-term immunity against gliomas.
Conclusions: These findings suggest that multiple low-dose intratumoral injections of CpG-ODN can eradicate intracranial gliomas possibly through mechanisms involving NK-mediated effector function.
Figures


Similar articles
-
Carbon nanotubes enhance CpG uptake and potentiate antiglioma immunity.Clin Cancer Res. 2011 Feb 15;17(4):771-82. doi: 10.1158/1078-0432.CCR-10-2444. Epub 2010 Nov 18. Clin Cancer Res. 2011. PMID: 21088258 Free PMC article.
-
TLR ligands in the local treatment of established intracerebral murine gliomas.J Immunol. 2008 Nov 15;181(10):6720-9. doi: 10.4049/jimmunol.181.10.6720. J Immunol. 2008. PMID: 18981089
-
Increase in tumor size following intratumoral injection of immunostimulatory CpG-containing oligonucleotides in a rat glioma model.Cancer Immunol Immunother. 2010 Apr;59(4):541-51. doi: 10.1007/s00262-009-0771-y. Epub 2009 Oct 2. Cancer Immunol Immunother. 2010. PMID: 19798500 Free PMC article.
-
Immunotherapeutic approach with oligodeoxynucleotides containing CpG motifs (CpG-ODN) in malignant glioma.Adv Exp Med Biol. 2012;746:95-108. doi: 10.1007/978-1-4614-3146-6_8. Adv Exp Med Biol. 2012. PMID: 22639162 Review.
-
Modulation of NK cell activity by CpG oligodeoxynucleotides.Immunol Res. 2007;39(1-3):15-21. doi: 10.1007/s12026-007-0066-3. Immunol Res. 2007. PMID: 17917052 Review.
Cited by
-
The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells.Immunology. 2013 Feb;138(2):105-15. doi: 10.1111/imm.12036. Immunology. 2013. PMID: 23216602 Free PMC article. Review.
-
The multidrug-resistance transporter Abcc3 protects NK cells from chemotherapy in a murine model of malignant glioma.Oncoimmunology. 2015 Oct 29;5(5):e1108513. doi: 10.1080/2162402X.2015.1108513. eCollection 2016 May. Oncoimmunology. 2015. PMID: 27467914 Free PMC article.
-
Production of bioactive soluble interleukin-15 in complex with interleukin-15 receptor alpha from a conditionally-replicating oncolytic HSV-1.PLoS One. 2013 Nov 27;8(11):e81768. doi: 10.1371/journal.pone.0081768. eCollection 2013. PLoS One. 2013. PMID: 24312353 Free PMC article.
-
Innovative and Promising Strategies to Enhance Effectiveness of Immunotherapy for CNS Tumors: Where Are We?Front Immunol. 2021 Jun 7;12:634031. doi: 10.3389/fimmu.2021.634031. eCollection 2021. Front Immunol. 2021. PMID: 34163465 Free PMC article. Review.
-
A new hope in immunotherapy for malignant gliomas: adoptive T cell transfer therapy.J Immunol Res. 2014;2014:326545. doi: 10.1155/2014/326545. Epub 2014 Jun 9. J Immunol Res. 2014. PMID: 25009822 Free PMC article. Review.
References
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. - PubMed
-
- Liau LM, Prins RM, Kiertscher SM, et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11:5515–25. - PubMed
-
- Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64:4973–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

