Mammalian target of rapamycin regulates miRNA-1 and follistatin in skeletal myogenesis
- PMID: 20566686
- PMCID: PMC2894448
- DOI: 10.1083/jcb.200912093
Mammalian target of rapamycin regulates miRNA-1 and follistatin in skeletal myogenesis
Abstract
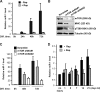
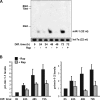
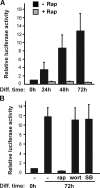
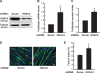
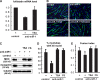
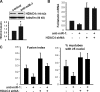
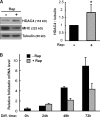
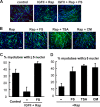
Mammalian target of rapamycin (mTOR) has emerged as a key regulator of skeletal muscle development by governing distinct stages of myogenesis, but the molecular pathways downstream of mTOR are not fully understood. In this study, we report that expression of the muscle-specific micro-RNA (miRNA) miR-1 is regulated by mTOR both in differentiating myoblasts and in mouse regenerating skeletal muscle. We have found that mTOR controls MyoD-dependent transcription of miR-1 through its upstream enhancer, most likely by regulating MyoD protein stability. Moreover, a functional pathway downstream of mTOR and miR-1 is delineated, in which miR-1 suppression of histone deacetylase 4 (HDAC4) results in production of follistatin and subsequent myocyte fusion. Collective evidence strongly suggests that follistatin is the long-sought mTOR-regulated fusion factor. In summary, our findings unravel for the first time a link between mTOR and miRNA biogenesis and identify an mTOR-miR-1-HDAC4-follistatin pathway that regulates myocyte fusion during myoblast differentiation in vitro and skeletal muscle regeneration in vivo.
Figures

Similar articles
-
IGF-II is regulated by microRNA-125b in skeletal myogenesis.J Cell Biol. 2011 Jan 10;192(1):69-81. doi: 10.1083/jcb.201007165. Epub 2011 Jan 3. J Cell Biol. 2011. PMID: 21200031 Free PMC article.
-
Deacetylase inhibitors increase muscle cell size by promoting myoblast recruitment and fusion through induction of follistatin.Dev Cell. 2004 May;6(5):673-84. doi: 10.1016/s1534-5807(04)00107-8. Dev Cell. 2004. PMID: 15130492
-
Guanidinoacetic Acid Regulates Myogenic Differentiation and Muscle Growth Through miR-133a-3p and miR-1a-3p Co-mediated Akt/mTOR/S6K Signaling Pathway.Int J Mol Sci. 2018 Sep 19;19(9):2837. doi: 10.3390/ijms19092837. Int J Mol Sci. 2018. PMID: 30235878 Free PMC article.
-
miR-378a-3p promotes differentiation and inhibits proliferation of myoblasts by targeting HDAC4 in skeletal muscle development.RNA Biol. 2016 Dec;13(12):1300-1309. doi: 10.1080/15476286.2016.1239008. Epub 2016 Sep 23. RNA Biol. 2016. PMID: 27661135 Free PMC article.
-
Mammalian target of rapamycin (mTOR) signaling network in skeletal myogenesis.J Biol Chem. 2012 Dec 21;287(52):43928-35. doi: 10.1074/jbc.R112.406942. Epub 2012 Oct 31. J Biol Chem. 2012. PMID: 23115234 Free PMC article. Review.
Cited by
-
The fine tuning of metabolism, autophagy and differentiation during in vitro myogenesis.Cell Death Dis. 2016 Mar 31;7(3):e2168. doi: 10.1038/cddis.2016.50. Cell Death Dis. 2016. PMID: 27031965 Free PMC article.
-
MBNL1 reverses the proliferation defect of skeletal muscle satellite cells in myotonic dystrophy type 1 by inhibiting autophagy via the mTOR pathway.Cell Death Dis. 2020 Jul 18;11(7):545. doi: 10.1038/s41419-020-02756-8. Cell Death Dis. 2020. PMID: 32683410 Free PMC article.
-
HDAC4 in cancer: A multitasking platform to drive not only epigenetic modifications.Front Mol Biosci. 2023 Jan 24;10:1116660. doi: 10.3389/fmolb.2023.1116660. eCollection 2023. Front Mol Biosci. 2023. PMID: 36762207 Free PMC article. Review.
-
Cell shape characteristics of human skeletal muscle cells as a predictor of myogenic competency: A new paradigm towards precision cell therapy.J Tissue Eng. 2023 Mar 16;14:20417314221139794. doi: 10.1177/20417314221139794. eCollection 2023 Jan-Dec. J Tissue Eng. 2023. PMID: 36949843 Free PMC article.
-
Analyses of MicroRNA and mRNA Expression Profiles Reveal the Crucial Interaction Networks and Pathways for Regulation of Chicken Breast Muscle Development.Front Genet. 2019 Mar 18;10:197. doi: 10.3389/fgene.2019.00197. eCollection 2019. Front Genet. 2019. PMID: 30936892 Free PMC article.
References
-
- Bodine S.C., Stitt T.N., Gonzalez M., Kline W.O., Stover G.L., Bauerlein R., Zlotchenko E., Scrimgeour A., Lawrence J.C., Glass D.J., Yancopoulos G.D. 2001. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 3:1014–1019 10.1038/ncb1101-1014 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

