Randomized trial of paclitaxel versus pegylated liposomal doxorubicin for advanced human immunodeficiency virus-associated Kaposi sarcoma: evidence of symptom palliation from chemotherapy
- PMID: 20564162
- PMCID: PMC3157242
- DOI: 10.1002/cncr.25362
Randomized trial of paclitaxel versus pegylated liposomal doxorubicin for advanced human immunodeficiency virus-associated Kaposi sarcoma: evidence of symptom palliation from chemotherapy
Abstract
Background: Paclitaxel and pegylated liposomal doxorubicin (PLD) are active cytotoxic agents for the treatment of human immunodeficiency virus (HIV)-associated Kaposi sarcoma (KS). A randomized trial comparing the efficacy and toxicity of paclitaxel and PLD was performed, and the effects of therapy on symptom palliation and quality of life were determined.
Methods: Patients with advanced HIV-associated KS were randomly assigned to receive paclitaxel at a dose of 100 mg/m2 intravenously (iv) every 2 weeks or PLD at a dose of 20 mg/m2 iv every 3 weeks. The KS Functional Assessment of HIV (FAHI) quality of life instrument was used before and after every other treatment cycle.
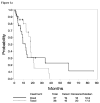
Results: The study included 73 analyzable patients enrolled between 1998 and 2002, including 36 in the paclitaxel arm and 37 in the PLD arm; 73% of patients received highly active antiretroviral therapy (HAART) and 32% had an undetectable viral load (<400 copies/mL). Treatment was associated with significant improvements in pain (P=.024) and swelling (P<.001). Of the 36 patients who reported that pain interfered with their normal work or activities at baseline, 25 (69%) improved. Of the 41 patients who reported swelling at baseline, 38 (93%) improved. Comparing the paclitaxel and PLD arms revealed comparable response rates (56% vs 46%; P=.49), median progression-free survival (17.5 months vs 12.2 months; P=.66), and 2-year survival rates (79% vs 78%; P=.75), but somewhat more grade 3 to 5 toxicity for paclitaxel (84% vs 66%; P=.077).
Conclusions: Treatment with either paclitaxel or PLD appears to produce significant improvements in pain and swelling in patients with advanced, symptomatic, HIV-associated KS treated in the HAART era.
Copyright (c) 2010 American Cancer Society.
Figures
Similar articles
-
Treatment of severe or progressive Kaposi's sarcoma in HIV-infected adults.Cochrane Database Syst Rev. 2014 Aug 13;8(8):CD003256. doi: 10.1002/14651858.CD003256.pub2. Cochrane Database Syst Rev. 2014. PMID: 25221796 Free PMC article. Review.
-
Treatment of severe or progressive Kaposi's sarcoma in HIV-infected adults.Cochrane Database Syst Rev. 2014;(9):CD003256. doi: 10.1002/14651858.CD003256.pub2. Cochrane Database Syst Rev. 2014. PMID: 25313415 Review.
-
Long-term prognosis of HIV-infected patients with Kaposi sarcoma treated with pegylated liposomal doxorubicin.Clin Infect Dis. 2008 Aug 1;47(3):410-7. doi: 10.1086/589865. Clin Infect Dis. 2008. PMID: 18582203 Clinical Trial.
-
A Pilot Study of Liposomal Doxorubicin Combined with Bevacizumab followed by Bevacizumab Monotherapy in Patients with Advanced Kaposi Sarcoma.Clin Cancer Res. 2019 Jul 15;25(14):4238-4247. doi: 10.1158/1078-0432.CCR-18-3528. Epub 2019 Apr 12. Clin Cancer Res. 2019. PMID: 30979736 Free PMC article.
-
Multicenter trial of low-dose paclitaxel in patients with advanced AIDS-related Kaposi sarcoma.Cancer. 2002 Jul 1;95(1):147-54. doi: 10.1002/cncr.10634. Cancer. 2002. PMID: 12115328 Clinical Trial.
Cited by
-
Human Gammaherpesvirus 8 Oncogenes Associated with Kaposi's Sarcoma.Int J Mol Sci. 2022 Jun 29;23(13):7203. doi: 10.3390/ijms23137203. Int J Mol Sci. 2022. PMID: 35806208 Free PMC article. Review.
-
Viral profiling identifies multiple subtypes of Kaposi's sarcoma.mBio. 2014 Sep 23;5(5):e01633-14. doi: 10.1128/mBio.01633-14. mBio. 2014. PMID: 25249280 Free PMC article.
-
Treatment of severe or progressive Kaposi's sarcoma in HIV-infected adults.Cochrane Database Syst Rev. 2014 Aug 13;8(8):CD003256. doi: 10.1002/14651858.CD003256.pub2. Cochrane Database Syst Rev. 2014. PMID: 25221796 Free PMC article. Review.
-
Excellent clinical outcomes and retention in care for adults with HIV-associated Kaposi sarcoma treated with systemic chemotherapy and integrated antiretroviral therapy in rural Malawi.J Int AIDS Soc. 2015 May 29;18(1):19929. doi: 10.7448/IAS.18.1.19929. eCollection 2015. J Int AIDS Soc. 2015. PMID: 26028156 Free PMC article.
-
Systemic management strategies for metastatic soft tissue sarcoma.Drugs. 2011 Nov 12;71(16):2115-29. doi: 10.2165/11594500-000000000-00000. Drugs. 2011. PMID: 22035513 Review.
References
-
- Engels EA, Pfeiffer RM, Goedert JJ, et al. Trends in cancer risk among people with AIDS in the United States 1980–2002. AIDS. 2006;20:1645–1654. - PubMed
-
- Jacobson LP, Yamashita TE, Detels R, et al. Impact of potent antiretroviral therapy on the incidence of Kaposi’s sarcoma and non-Hodgkin’s lymphomas among HIV-1-infected individuals. Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 1999;21 (suppl 1):S34–S41. - PubMed
-
- Jones JL, Hanson DL, Dworkin MS, et al. Incidence and trends in Kaposi’s sarcoma in the era of effective antiretroviral therapy. J Acquir Immune Defic Syndr. 2000;24:270–274. - PubMed
-
- Sparano JA, Anand K, Desai J, et al. Effect of highly active antiretroviral therapy on the incidence of HIV-associated malignancies at an urban medical center. J Acquir Immune Defic Syndr. 1999;21(suppl 1):S18–S22. - PubMed
-
- Carrieri MP, Pradier C, Piselli P, et al. Reduced incidence of Kaposi’s sarcoma and of systemic non-Hodgkin’s lymphoma in HIV-infected individuals treated with highly active antiretroviral therapy. Int J Cancer. 2003;103:142–144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical