Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes
- PMID: 20562852
- PMCID: PMC2920067
- DOI: 10.1038/nature09253
Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes
Abstract
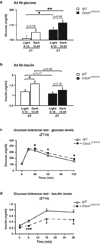
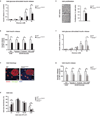
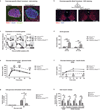
The molecular clock maintains energy constancy by producing circadian oscillations of rate-limiting enzymes involved in tissue metabolism across the day and night. During periods of feeding, pancreatic islets secrete insulin to maintain glucose homeostasis, and although rhythmic control of insulin release is recognized to be dysregulated in humans with diabetes, it is not known how the circadian clock may affect this process. Here we show that pancreatic islets possess self-sustained circadian gene and protein oscillations of the transcription factors CLOCK and BMAL1. The phase of oscillation of islet genes involved in growth, glucose metabolism and insulin signalling is delayed in circadian mutant mice, and both Clock and Bmal1 (also called Arntl) mutants show impaired glucose tolerance, reduced insulin secretion and defects in size and proliferation of pancreatic islets that worsen with age. Clock disruption leads to transcriptome-wide alterations in the expression of islet genes involved in growth, survival and synaptic vesicle assembly. Notably, conditional ablation of the pancreatic clock causes diabetes mellitus due to defective beta-cell function at the very latest stage of stimulus-secretion coupling. These results demonstrate a role for the beta-cell clock in coordinating insulin secretion with the sleep-wake cycle, and reveal that ablation of the pancreatic clock can trigger the onset of diabetes mellitus.
Figures

Comment in
-
Metabolism: Tick, tock, a beta-cell clock.Nature. 2010 Jul 29;466(7306):571-2. doi: 10.1038/466571a. Nature. 2010. PMID: 20671699 Free PMC article.
Similar articles
-
Metabolism: Tick, tock, a beta-cell clock.Nature. 2010 Jul 29;466(7306):571-2. doi: 10.1038/466571a. Nature. 2010. PMID: 20671699 Free PMC article.
-
Induction of Core Circadian Clock Transcription Factor Bmal1 Enhances β-Cell Function and Protects Against Obesity-Induced Glucose Intolerance.Diabetes. 2021 Jan;70(1):143-154. doi: 10.2337/db20-0192. Epub 2020 Oct 21. Diabetes. 2021. PMID: 33087455 Free PMC article.
-
Bmal1 is required for beta cell compensatory expansion, survival and metabolic adaptation to diet-induced obesity in mice.Diabetologia. 2016 Apr;59(4):734-43. doi: 10.1007/s00125-015-3859-2. Epub 2016 Jan 13. Diabetologia. 2016. PMID: 26762333 Free PMC article.
-
[The roles of clock genes in obesity].Nihon Rinsho. 2013 Feb;71(2):244-8. Nihon Rinsho. 2013. PMID: 23631200 Review. Japanese.
-
Clock genes, pancreatic function, and diabetes.Trends Mol Med. 2014 Dec;20(12):685-93. doi: 10.1016/j.molmed.2014.10.007. Epub 2014 Nov 5. Trends Mol Med. 2014. PMID: 25457619 Free PMC article. Review.
Cited by
-
Circadian Regulation of the Pancreatic Beta Cell.Endocrinology. 2021 Sep 1;162(9):bqab089. doi: 10.1210/endocr/bqab089. Endocrinology. 2021. PMID: 33914056 Free PMC article. Review.
-
Genome-wide circadian regulation: A unique system for computational biology.Comput Struct Biotechnol J. 2020 Jul 10;18:1914-1924. doi: 10.1016/j.csbj.2020.07.002. eCollection 2020. Comput Struct Biotechnol J. 2020. PMID: 32774786 Free PMC article. Review.
-
Long-term effects of melatonin on quality of life and sleep in haemodialysis patients (Melody study): a randomized controlled trial.Br J Clin Pharmacol. 2013 Nov;76(5):668-79. doi: 10.1111/bcp.12093. Br J Clin Pharmacol. 2013. PMID: 23432361 Free PMC article. Clinical Trial.
-
A real-time measurement system for gene expression rhythms from deep tissues of freely moving mice under light-dark conditions.Biochem Biophys Rep. 2022 Sep 18;32:101344. doi: 10.1016/j.bbrep.2022.101344. eCollection 2022 Dec. Biochem Biophys Rep. 2022. PMID: 36160030 Free PMC article.
-
DNA damage shifts circadian clock time via Hausp-dependent Cry1 stabilization.Elife. 2015 Mar 10;4:e04883. doi: 10.7554/eLife.04883. Elife. 2015. PMID: 25756610 Free PMC article.
References
-
- Panda S, et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. - PubMed
-
- Rutter J, Reick M, McKnight SL. Metabolism and the control of circadian rhythms. Annu Rev Biochem. 2002;71:307–331. - PubMed
-
- Polonsky KS, et al. Abnormal patterns of insulin secretion in non-insulin-dependent diabetes mellitus. N Engl J Med. 1988;318:1231–1239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

