TFIIA and the transactivator Rap1 cooperate to commit TFIID for transcription initiation
- PMID: 20559389
- PMCID: PMC2900199
- DOI: 10.1038/nature09080
TFIIA and the transactivator Rap1 cooperate to commit TFIID for transcription initiation
Abstract
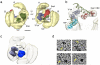
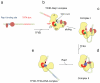
Transcription of eukaryotic messenger RNA (mRNA) encoding genes by RNA polymerase II (Pol II) is triggered by the binding of transactivating proteins to enhancer DNA, which stimulates the recruitment of general transcription factors (TFIIA, B, D, E, F, H) and Pol II on the cis-linked promoter, leading to pre-initiation complex formation and transcription. In TFIID-dependent activation pathways, this general transcription factor containing TATA-box-binding protein is first recruited on the promoter through interaction with activators and cooperates with TFIIA to form a committed pre-initiation complex. However, neither the mechanisms by which activation signals are communicated between these factors nor the structural organization of the activated pre-initiation complex are known. Here we used cryo-electron microscopy to determine the architecture of nucleoprotein complexes composed of TFIID, TFIIA, the transcriptional activator Rap1 and yeast enhancer-promoter DNA. These structures revealed the mode of binding of Rap1 and TFIIA to TFIID, as well as a reorganization of TFIIA induced by its interaction with Rap1. We propose that this change in position increases the exposure of TATA-box-binding protein within TFIID, consequently enhancing its ability to interact with the promoter. A large Rap1-dependent DNA loop forms between the activator-binding site and the proximal promoter region. This loop is topologically locked by a TFIIA-Rap1 protein bridge that folds over the DNA. These results highlight the role of TFIIA in transcriptional activation, define a molecular mechanism for enhancer-promoter communication and provide structural insights into the pathways of intramolecular communication that convey transcription activation signals through the TFIID complex.
Figures


Similar articles
-
Direct TFIIA-TFIID protein contacts drive budding yeast ribosomal protein gene transcription.J Biol Chem. 2013 Aug 9;288(32):23273-94. doi: 10.1074/jbc.M113.486829. Epub 2013 Jun 27. J Biol Chem. 2013. PMID: 23814059 Free PMC article.
-
A transcription factor IIA-binding site differentially regulates RNA polymerase II-mediated transcription in a promoter context-dependent manner.J Biol Chem. 2017 Jul 14;292(28):11873-11885. doi: 10.1074/jbc.M116.770412. Epub 2017 May 24. J Biol Chem. 2017. PMID: 28539359 Free PMC article.
-
Structure of promoter-bound TFIID and model of human pre-initiation complex assembly.Nature. 2016 Mar 31;531(7596):604-9. doi: 10.1038/nature17394. Epub 2016 Mar 23. Nature. 2016. PMID: 27007846 Free PMC article.
-
Structural insights into assembly of transcription preinitiation complex.Curr Opin Struct Biol. 2022 Aug;75:102404. doi: 10.1016/j.sbi.2022.102404. Epub 2022 Jun 11. Curr Opin Struct Biol. 2022. PMID: 35700575 Review.
-
Mechanisms of transcriptional activation in vivo: two steps forward.Trends Genet. 1996 Aug;12(8):311-5. doi: 10.1016/0168-9525(96)10028-7. Trends Genet. 1996. PMID: 8783941 Review.
Cited by
-
Multiple roles of the general regulatory factor Abf1 in yeast ribosome biogenesis.Curr Genet. 2017 Feb;63(1):65-68. doi: 10.1007/s00294-016-0621-3. Epub 2016 Jun 4. Curr Genet. 2017. PMID: 27262581 Review.
-
Context-dependent function of the transcriptional regulator Rap1 in gene silencing and activation in Saccharomyces cerevisiae.Proc Natl Acad Sci U S A. 2023 Oct 3;120(40):e2304343120. doi: 10.1073/pnas.2304343120. Epub 2023 Sep 28. Proc Natl Acad Sci U S A. 2023. PMID: 37769255 Free PMC article.
-
Gene-specific transcription activation via long-range allosteric shape-shifting.Biochem J. 2011 Oct 1;439(1):15-25. doi: 10.1042/BJ20110972. Biochem J. 2011. PMID: 21916844 Free PMC article. Review.
-
Hypomorphic Pathogenic Variants in TAF13 Are Associated with Autosomal-Recessive Intellectual Disability and Microcephaly.Am J Hum Genet. 2017 Mar 2;100(3):555-561. doi: 10.1016/j.ajhg.2017.01.032. Am J Hum Genet. 2017. PMID: 28257693 Free PMC article.
-
Molecular structure of promoter-bound yeast TFIID.Nat Commun. 2018 Nov 7;9(1):4666. doi: 10.1038/s41467-018-07096-y. Nat Commun. 2018. PMID: 30405110 Free PMC article.
References
-
- Burley SK, Roeder RG. Biochemistry and structural biology of transcription factor IID (TFIID) Annu Rev Biochem. 1996;65:769–799. - PubMed
-
- Verrijzer CP, Tjian R. TAFs mediate transcriptional activation and promoter selectivity. Trends Biochem Sci. 1996;21:338–342. - PubMed
-
- Jacq X, et al. Human TAFII30 is present in a distinct TFIID complex and is required for transcriptional activation by the estrogen receptor. Cell. 1994;79:107–117. - PubMed
-
- Lieberman PM, Berk AJ. A mechanism for TAFs in transcriptional activation: activation domain enhancement of TFIID-TFIIA--promoter DNA complex formation. Genes Dev. 1994;8:995–1006. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

