PD-L1 has distinct functions in hematopoietic and nonhematopoietic cells in regulating T cell responses during chronic infection in mice
- PMID: 20551512
- PMCID: PMC2898584
- DOI: 10.1172/JCI40040
PD-L1 has distinct functions in hematopoietic and nonhematopoietic cells in regulating T cell responses during chronic infection in mice
Abstract
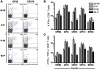
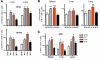
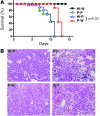
The inhibitory receptor programmed death 1 (PD-1) is upregulated on antigen-specific CD8+ T cells during persistent viral infections. Interaction with PD-1 ligand 1 (PD-L1) contributes to functional exhaustion of responding T cells and may limit immunopathology during infection. PD-L1 is expressed on both hematopoietic and nonhematopoietic cells in tissues. However, the exact roles of PD-L1 on hematopoietic versus nonhematopoietic cells in modulating immune responses are unclear. Here we used bone marrow chimeric mice to examine the effects of PD-L1 deficiency in hematopoietic or nonhematopoietic cells during lymphocytic choriomeningitis virus clone 13 (LCMV CL-13) infection. We found that PD-L1 expression on hematopoietic cells inhibited CD8+ T cell numbers and function after LCMV CL-13 infection. In contrast, PD-L1 expression on nonhematopoietic cells limited viral clearance and immunopathology in infected tissues. Together, these data demonstrate that there are distinct roles for PD-L1 on hematopoietic and nonhematopoietic cells in regulating CD8+ T cell responses and viral clearance during chronic viral infection.
Figures

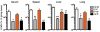

Similar articles
-
PD-L1 Checkpoint Inhibition Narrows the Antigen-Specific T Cell Receptor Repertoire in Chronic Lymphocytic Choriomeningitis Virus Infection.J Virol. 2020 Aug 31;94(18):e00795-20. doi: 10.1128/JVI.00795-20. Print 2020 Aug 31. J Virol. 2020. PMID: 32641478 Free PMC article.
-
Aplastic anemia rescued by exhaustion of cytokine-secreting CD8+ T cells in persistent infection with lymphocytic choriomeningitis virus.J Exp Med. 1998 Jun 1;187(11):1903-20. doi: 10.1084/jem.187.11.1903. J Exp Med. 1998. PMID: 9607930 Free PMC article.
-
Interplay between regulatory T cells and PD-1 in modulating T cell exhaustion and viral control during chronic LCMV infection.J Exp Med. 2014 Aug 25;211(9):1905-18. doi: 10.1084/jem.20132577. Epub 2014 Aug 11. J Exp Med. 2014. PMID: 25113973 Free PMC article.
-
CD8 T cell dysfunction during chronic viral infection.Curr Opin Immunol. 2007 Aug;19(4):408-15. doi: 10.1016/j.coi.2007.06.004. Epub 2007 Jul 25. Curr Opin Immunol. 2007. PMID: 17656078 Review.
-
IL-10: achieving balance during persistent viral infection.Curr Top Microbiol Immunol. 2014;380:129-44. doi: 10.1007/978-3-662-43492-5_6. Curr Top Microbiol Immunol. 2014. PMID: 25004816 Review.
Cited by
-
Genetic absence of PD-L1 does not restore CD8+ T cell function during respiratory virus infection and delays virus clearance.J Virol. 2024 Oct 22;98(10):e0079724. doi: 10.1128/jvi.00797-24. Epub 2024 Sep 23. J Virol. 2024. PMID: 39311697
-
Predictive Biomarkers and Resistance Mechanisms of Checkpoint Inhibitors in Malignant Solid Tumors.Int J Mol Sci. 2024 Sep 6;25(17):9659. doi: 10.3390/ijms25179659. Int J Mol Sci. 2024. PMID: 39273605 Free PMC article. Review.
-
Harnessing IL-2 for immunotherapy against cancer and chronic infection: a historical perspective and emerging trends.Exp Mol Med. 2024 Sep;56(9):1900-1908. doi: 10.1038/s12276-024-01301-3. Epub 2024 Sep 2. Exp Mol Med. 2024. PMID: 39218982 Free PMC article. Review.
-
Lymphatic system regulation of anti-cancer immunity and metastasis.Front Immunol. 2024 Aug 15;15:1449291. doi: 10.3389/fimmu.2024.1449291. eCollection 2024. Front Immunol. 2024. PMID: 39211044 Free PMC article. Review.
-
Distinct fibroblast functions associated with fibrotic and immune-mediated inflammatory diseases and their implications for therapeutic development.F1000Res. 2024 Jan 10;13:54. doi: 10.12688/f1000research.143472.1. eCollection 2024. F1000Res. 2024. PMID: 38681509 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

