Crosstalk between decidual NK and CD14+ myelomonocytic cells results in induction of Tregs and immunosuppression
- PMID: 20547831
- PMCID: PMC2900704
- DOI: 10.1073/pnas.1001749107
Crosstalk between decidual NK and CD14+ myelomonocytic cells results in induction of Tregs and immunosuppression
Abstract
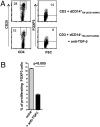
Regulatory T cells (Tregs) are thought to play a major role in pregnancy by inhibiting the maternal immune system and preventing fetal rejection. In decidual tissues, NK cells (dNK) reside in close contact with particular myelomonocytic CD14(+) (dCD14(+)) cells. Here we show that the interaction between dNK and dCD14(+) cells results in induction of Tregs. The interaction is mediated by soluble factors as shown by transwell experiments, and the prominent role of IFN-gamma is revealed by the effect of a neutralizing monoclonal antibody. Following interaction with dNK cells, dCD14(+) cells express indoleamine 2,3-dioxygenase (IDO), which, in turn, induces Tregs. Notably, unlike peripheral blood NK (pNK) cells, dNK cells are resistant to inhibition by the IDO metabolite L-kynurenine. "Conditioned" dCD14(+) cells also may induce Tregs through transforming growth factor-beta (TGF-beta) production or CTLA-4-mediated interactions, as indicated by the effect of specific neutralizing Abs. Remarkably, only the interaction between dNK and dCD14(+) cells results in Treg induction, whereas other coculture combinations involving either NK or CD14(+) cells isolated from peripheral blood are ineffective. Our study provides interesting clues to understanding how the crosstalk between decidual NK and CD14(+) cells may initiate a process that leads to Treg induction and immunosuppression. Along this line, it is conceivable that an impaired function of these cells may result in pregnancy failure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




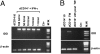

Similar articles
-
Natural killer cells in human pregnancy.J Reprod Immunol. 2013 Mar;97(1):14-9. doi: 10.1016/j.jri.2012.10.008. J Reprod Immunol. 2013. PMID: 23432867 Review.
-
Stromal cells from human decidua exert a strong inhibitory effect on NK cell function and dendritic cell differentiation.PLoS One. 2014 Feb 20;9(2):e89006. doi: 10.1371/journal.pone.0089006. eCollection 2014. PLoS One. 2014. PMID: 24586479 Free PMC article.
-
Effect of Indoleamine 2,3-Dioxygenase Expressed in HTR-8/SVneo Cells on Decidual NK Cell Cytotoxicity.Am J Reprod Immunol. 2016 May;75(5):519-28. doi: 10.1111/aji.12481. Epub 2016 Jan 18. Am J Reprod Immunol. 2016. PMID: 26782048
-
Pigment epithelium-derived factor, a novel decidual natural killer cells-derived factor, protects decidual stromal cells via anti-inflammation and anti-apoptosis in early pregnancy.Hum Reprod. 2020 Jul 1;35(7):1537-1552. doi: 10.1093/humrep/deaa118. Hum Reprod. 2020. PMID: 32544239
-
Human Innate Lymphoid Cells: Their Functional and Cellular Interactions in Decidua.Front Immunol. 2018 Aug 14;9:1897. doi: 10.3389/fimmu.2018.01897. eCollection 2018. Front Immunol. 2018. PMID: 30154799 Free PMC article. Review.
Cited by
-
Role of Genital Tract Bacteria in Promoting Endometrial Health in Cattle.Microorganisms. 2022 Nov 12;10(11):2238. doi: 10.3390/microorganisms10112238. Microorganisms. 2022. PMID: 36422307 Free PMC article.
-
Endometrial Immune Dysfunction in Recurrent Pregnancy Loss.Int J Mol Sci. 2019 Oct 26;20(21):5332. doi: 10.3390/ijms20215332. Int J Mol Sci. 2019. PMID: 31717776 Free PMC article. Review.
-
Role of Natural Killer Cells during Pregnancy and Related Complications.Biomolecules. 2022 Jan 4;12(1):68. doi: 10.3390/biom12010068. Biomolecules. 2022. PMID: 35053216 Free PMC article. Review.
-
Trophoblast induces monocyte differentiation into CD14+/CD16+ macrophages.Am J Reprod Immunol. 2014 Sep;72(3):270-84. doi: 10.1111/aji.12288. Epub 2014 Jul 3. Am J Reprod Immunol. 2014. PMID: 24995492 Free PMC article.
-
Circulating presepsin (soluble CD14 subtype) as a marker of host response in patients with severe sepsis or septic shock: data from the multicenter, randomized ALBIOS trial.Intensive Care Med. 2015 Jan;41(1):12-20. doi: 10.1007/s00134-014-3514-2. Epub 2014 Oct 16. Intensive Care Med. 2015. PMID: 25319385 Clinical Trial.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

