Functional basis for complement evasion by staphylococcal superantigen-like 7
- PMID: 20545943
- PMCID: PMC2941559
- DOI: 10.1111/j.1462-5822.2010.01486.x
Functional basis for complement evasion by staphylococcal superantigen-like 7
Abstract
The human pathogen Staphylococcus aureus has a plethora of virulence factors that promote its colonization and survival in the host. Among such immune modulators are staphylococcal superantigen-like (SSL) proteins, comprising a family of 14 small, secreted molecules that seem to interfere with the host innate immune system. SSL7 has been described to bind immunoglobulin A (IgA) and complement C5, thereby inhibiting IgA-FcαRI binding and serum killing of Escherichia coli. As C5a generation, in contrast to C5b-9-mediated lysis, is crucial for immune defence against staphylococci, we investigated the impact of SSL7 on staphylococcal-induced C5a-mediated effects. Here, we show that SSL7 inhibits C5a generation induced by staphylococcal opsonization, slightly enhanced by its IgA-binding capacity. Moreover, we demonstrate a strong protective activity of SSL7 against staphylococcal clearance in human whole blood. SSL7 strongly inhibited the C5a-induced phagocytosis of S. aureus and oxidative burst in an in vitro whole-blood inflammation model. Furthermore, we found that SSL7 affects all three pathways of complement activation and inhibits the cleavage of C5 by interference of its binding to C5 convertases. Finally, SSL7 effects were also demonstrated in vivo. In a murine model of immune complex peritonitis, SSL7 abrogated the C5a-driven influx of neutrophils in mouse peritoneum.
© 2010 Blackwell Publishing Ltd.
Figures
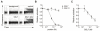


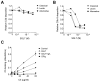

Similar articles
-
Full functional activity of SSL7 requires binding of both complement C5 and IgA.Immunol Cell Biol. 2013 Aug;91(7):469-76. doi: 10.1038/icb.2013.28. Epub 2013 Jun 25. Immunol Cell Biol. 2013. PMID: 23797068
-
Structural basis for inhibition of complement C5 by the SSL7 protein from Staphylococcus aureus.Proc Natl Acad Sci U S A. 2010 Feb 23;107(8):3681-6. doi: 10.1073/pnas.0910565107. Epub 2010 Feb 4. Proc Natl Acad Sci U S A. 2010. PMID: 20133685 Free PMC article.
-
Structural basis for evasion of IgA immunity by Staphylococcus aureus revealed in the complex of SSL7 with Fc of human IgA1.Proc Natl Acad Sci U S A. 2007 Sep 18;104(38):15051-6. doi: 10.1073/pnas.0706028104. Epub 2007 Sep 11. Proc Natl Acad Sci U S A. 2007. PMID: 17848512 Free PMC article.
-
Immune evasion by staphylococci.Nat Rev Microbiol. 2005 Dec;3(12):948-58. doi: 10.1038/nrmicro1289. Nat Rev Microbiol. 2005. PMID: 16322743 Review.
-
[Modulation of Host Immune System by Staphylococcal Superantigen-like (SSL) Proteins].Yakugaku Zasshi. 2021;141(4):579-589. doi: 10.1248/yakushi.20-00236. Yakugaku Zasshi. 2021. PMID: 33790123 Review. Japanese.
Cited by
-
Functional Characterization of Alternative and Classical Pathway C3/C5 Convertase Activity and Inhibition Using Purified Models.Front Immunol. 2018 Jul 23;9:1691. doi: 10.3389/fimmu.2018.01691. eCollection 2018. Front Immunol. 2018. PMID: 30083158 Free PMC article.
-
Pre-existing antibody-mediated adverse effects prevent the clinical development of a bacterial anti-inflammatory protein.Dis Model Mech. 2020 Sep 28;13(9):dmm045534. doi: 10.1242/dmm.045534. Dis Model Mech. 2020. PMID: 32471891 Free PMC article. Clinical Trial.
-
Determinants of bacterial survival and proliferation in blood.FEMS Microbiol Rev. 2024 May 8;48(3):fuae013. doi: 10.1093/femsre/fuae013. FEMS Microbiol Rev. 2024. PMID: 38734892 Free PMC article. Review.
-
Epic Immune Battles of History: Neutrophils vs. Staphylococcus aureus.Front Cell Infect Microbiol. 2017 Jun 30;7:286. doi: 10.3389/fcimb.2017.00286. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28713774 Free PMC article. Review.
-
Hyperglycemia inhibits complement-mediated immunological control of S. aureus in a rat model of peritonitis.J Diabetes Res. 2014;2014:762051. doi: 10.1155/2014/762051. Epub 2014 Dec 31. J Diabetes Res. 2014. PMID: 25610878 Free PMC article.
References
-
- Arcus VL, Langley R, Proft T, Fraser JD, Baker EN. The Three-dimensional structure of a superantigen-like protein, SET3, from a pathogenicity island of the Staphylococcus aureus genome. J Biol Chem. 2002;277:32274–32281. - PubMed
-
- Bestebroer J, Poppelier MJ, Ulfman LH, Lenting PJ, Denis CV, van Kessel KP. Staphylococcal superantigen-like 5 binds PSGL-1 and inhibits P-selectin-mediated neutrophil rolling. Blood. 2007;109:2936–2943. el al. - PubMed
-
- Beutler B. Innate immunity: an overview. Mol Immunol. 2004;40:845–859. - PubMed
-
- Boel E, Verlaan S, Poppelier MJ, Westerdaal NA, van Strijp JA, Logtenberg T. Functional human monoclonal antibodies of all isotypes constructed from phage display library-derived single-chain Fv antibody fragments. J Immunol Methods. 2000;239:153–166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

