T cells enhance stem-like properties and conditional malignancy in gliomas
- PMID: 20539758
- PMCID: PMC2881867
- DOI: 10.1371/journal.pone.0010974
T cells enhance stem-like properties and conditional malignancy in gliomas
Abstract
Background: Small populations of highly tumorigenic stem-like cells (cancer stem cells; CSCs) can exist within, and uniquely regenerate cancers including malignant brain tumors (gliomas). Many aspects of glioma CSCs (GSCs), however, have been characterized in non-physiological settings.
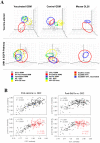
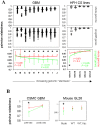
Methods: We found gene expression similarity superiorly defined glioma "stemness", and revealed that GSC similarity increased with lower tumor grade. Using this method, we examined stemness in human grade IV gliomas (GBM) before and after dendritic cell (DC) vaccine therapy. This was followed by gene expression, phenotypic and functional analysis of murine GL26 tumors recovered from nude, wild-type, or DC-vaccinated host brains.
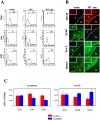
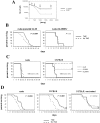
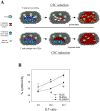
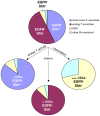
Results: GSC similarity was specifically increased in post-vaccine GBMs, and correlated best to vaccine-altered gene expression and endogenous anti-tumor T cell activity. GL26 analysis confirmed immune alterations, specific acquisition of stem cell markers, specifically enhanced sensitivity to anti-stem drug (cyclopamine), and enhanced tumorigenicity in wild-type hosts, in tumors in proportion to anti-tumor T cell activity. Nevertheless, vaccine-exposed GL26 cells were no more tumorigenic than parental GL26 in T cell-deficient hosts, though they otherwise appeared similar to GSCs enriched by chemotherapy. Finally, vaccine-exposed GBM and GL26 exhibited relatively homogeneous expression of genes expressed in progenitor cells and/or differentiation.
Conclusions: T cell activity represents an inducible physiological process capable of proportionally enriching GSCs in human and mouse gliomas. Stem-like gliomas enriched by strong T cell activity, however, may differ from other GSCs in that their stem-like properties may be disassociated from increased tumor malignancy and heterogeneity under specific host immune conditions.
Conflict of interest statement
Figures

Similar articles
-
Glioma stem cells are more aggressive in recurrent tumors with malignant progression than in the primary tumor, and both can be maintained long-term in vitro.BMC Cancer. 2008 Oct 22;8:304. doi: 10.1186/1471-2407-8-304. BMC Cancer. 2008. PMID: 18940013 Free PMC article.
-
Overexpression of ZNF217 in glioblastoma contributes to the maintenance of glioma stem cells regulated by hypoxia-inducible factors.Lab Invest. 2011 Jul;91(7):1068-78. doi: 10.1038/labinvest.2011.56. Epub 2011 Apr 11. Lab Invest. 2011. PMID: 21483406
-
Enhancer of Zeste 2 (EZH2) is up-regulated in malignant gliomas and in glioma stem-like cells.Neuropathol Appl Neurobiol. 2011 Jun;37(4):381-94. doi: 10.1111/j.1365-2990.2010.01132.x. Neuropathol Appl Neurobiol. 2011. PMID: 20946108
-
New advances of microRNAs in glioma stem cells, with special emphasis on aberrant methylation of microRNAs.J Cell Physiol. 2014 Sep;229(9):1141-7. doi: 10.1002/jcp.24540. J Cell Physiol. 2014. PMID: 24374932 Review.
-
The Glioma Stem Cell Model in the Era of Single-Cell Genomics.Cancer Cell. 2020 May 11;37(5):630-636. doi: 10.1016/j.ccell.2020.04.001. Cancer Cell. 2020. PMID: 32396858 Review.
Cited by
-
Cancer stem cell hypotheses: impact on modern molecular physiology and pharmacology research.J Biosci. 2011 Dec;36(5):957-61. doi: 10.1007/s12038-011-9155-5. J Biosci. 2011. PMID: 22116294 Review.
-
Exploitation of adaptive evolution in glioma treatment.CNS Oncol. 2013 Mar;2(2):171-9. doi: 10.2217/cns.12.46. CNS Oncol. 2013. PMID: 23977426 Free PMC article. Review.
-
How to conjugate the stemness marker ALDH1A1 with tumor angiogenesis, progression, and drug resistance.Cancer Drug Resist. 2020 Mar 19;3(1):26-37. doi: 10.20517/cdr.2019.70. eCollection 2020. Cancer Drug Resist. 2020. PMID: 35582039 Free PMC article. Review.
-
Cancer stem cells: perspectives for therapeutic targeting.Cancer Immunol Immunother. 2015 Jan;64(1):91-7. doi: 10.1007/s00262-014-1592-1. Epub 2014 Aug 8. Cancer Immunol Immunother. 2015. PMID: 25104304 Free PMC article. Review.
-
Glioma immunotherapy enhancement and CD8-specific sialic acid cleavage by isocitrate dehydrogenase (IDH)-1.Oncogene. 2023 Jun;42(25):2088-2098. doi: 10.1038/s41388-023-02713-7. Epub 2023 May 9. Oncogene. 2023. PMID: 37161052 Free PMC article.
References
-
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, et al. Identification of human brain tumour initiating cells. Nature. 2004;432 - PubMed
-
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. - PubMed
-
- Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. N Engl J Med. 2005;353:811–822. - PubMed
-
- Piccirillo SGM, Reynolds BA, Zanetti N, Lamorte G, Binda E, et al. Bone morphogenetic proteins inhibit the tumorigenic potential of human bran tumour-initiating cells. Nature. 2006;444:761–765. - PubMed
-
- Ridgway J, Zhang G, Wu Y, Stawicki S, Liang W-C, et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083–1087. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

