Up-regulation of the ATPase inhibitory factor 1 (IF1) of the mitochondrial H+-ATP synthase in human tumors mediates the metabolic shift of cancer cells to a Warburg phenotype
- PMID: 20538613
- PMCID: PMC2919093
- DOI: 10.1074/jbc.M110.146480
Up-regulation of the ATPase inhibitory factor 1 (IF1) of the mitochondrial H+-ATP synthase in human tumors mediates the metabolic shift of cancer cells to a Warburg phenotype
Abstract
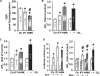
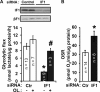
The H(+)-ATP synthase is a reversible engine of mitochondria that synthesizes or hydrolyzes ATP upon changes in cell physiology. ATP synthase dysfunction is involved in the onset and progression of diverse human pathologies. During ischemia, the ATP hydrolytic activity of the enzyme is inhibited by the ATPase inhibitory factor 1 (IF1). The expression of IF1 in human tissues and its participation in the development of human pathology are unknown. Here, we have developed monoclonal antibodies against human IF1 and determined its expression in paired normal and tumor biopsies of human carcinomas. We show that the relative mitochondrial content of IF1 increases significantly in carcinomas, suggesting the participation of IF1 in oncogenesis. The expression of IF1 varies significantly in cancer cell lines. To investigate the functional activity of IF1 in cancer, we have manipulated its cellular content. Overexpression of IF1 or of its pH-insensitive H49K mutant in cells that express low levels of IF1 triggers the up-regulation of aerobic glycolysis and the inhibition of oxidative phosphorylation with concurrent mitochondrial hyperpolarization. Treatment of the cells with the H(+)-ATP synthase inhibitor oligomycin mimicked the effects of IF1 overexpression. Conversely, small interfering RNA-mediated silencing of IF1 in cells that express high levels of IF1 promotes the down-regulation of aerobic glycolysis and the increase in oxidative phosphorylation. Overall, these findings support that the mitochondrial content of IF1 controls the activity of oxidative phosphorylation mediating the shift of cancer cells to an enhanced aerobic glycolysis, thus supporting an oncogenic role for the de-regulated expression of IF1 in cancer.
Figures

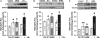
Similar articles
-
The ATPase Inhibitory Factor 1 (IF1): A master regulator of energy metabolism and of cell survival.Biochim Biophys Acta. 2016 Aug;1857(8):1167-1182. doi: 10.1016/j.bbabio.2016.02.004. Epub 2016 Feb 12. Biochim Biophys Acta. 2016. PMID: 26876430
-
PKA Phosphorylates the ATPase Inhibitory Factor 1 and Inactivates Its Capacity to Bind and Inhibit the Mitochondrial H(+)-ATP Synthase.Cell Rep. 2015 Sep 29;12(12):2143-55. doi: 10.1016/j.celrep.2015.08.052. Epub 2015 Sep 17. Cell Rep. 2015. PMID: 26387949
-
Glucose-modulated mitochondria adaptation in tumor cells: a focus on ATP synthase and inhibitor Factor 1.Int J Mol Sci. 2012;13(2):1933-1950. doi: 10.3390/ijms13021933. Epub 2012 Feb 10. Int J Mol Sci. 2012. PMID: 22408432 Free PMC article.
-
Regulation of the H+-ATP synthase by IF1: a role in mitohormesis.Cell Mol Life Sci. 2017 Jun;74(12):2151-2166. doi: 10.1007/s00018-017-2462-8. Epub 2017 Feb 6. Cell Mol Life Sci. 2017. PMID: 28168445 Free PMC article. Review.
-
The F1Fo-ATPase inhibitor, IF1, is a critical regulator of energy metabolism in cancer cells.Biochem Soc Trans. 2021 Apr 30;49(2):815-827. doi: 10.1042/BST20200742. Biochem Soc Trans. 2021. PMID: 33929490 Review.
Cited by
-
Pyruvate kinase M2 and the mitochondrial ATPase Inhibitory Factor 1 provide novel biomarkers of dermatomyositis: a metabolic link to oncogenesis.J Transl Med. 2017 Feb 10;15(1):29. doi: 10.1186/s12967-017-1136-5. J Transl Med. 2017. PMID: 28183315 Free PMC article.
-
ATP synthase inhibitory factor subunit 1 regulates islet β-cell function via repression of mitochondrial homeostasis.Lab Invest. 2022 Jan;102(1):69-79. doi: 10.1038/s41374-021-00670-x. Epub 2021 Oct 4. Lab Invest. 2022. PMID: 34608240 Free PMC article.
-
Phosphatidylethanolamine deficiency in Mammalian mitochondria impairs oxidative phosphorylation and alters mitochondrial morphology.J Biol Chem. 2013 Feb 8;288(6):4158-73. doi: 10.1074/jbc.M112.434183. Epub 2012 Dec 18. J Biol Chem. 2013. PMID: 23250747 Free PMC article.
-
ATPase inhibitory factor 1 expression is an independent prognostic factor in non-small cell lung cancer.Am J Cancer Res. 2016 May 1;6(5):1141-8. eCollection 2016. Am J Cancer Res. 2016. PMID: 27294006 Free PMC article.
-
Impaired mitochondrial metabolism and mammary carcinogenesis.J Mammary Gland Biol Neoplasia. 2013 Mar;18(1):75-87. doi: 10.1007/s10911-012-9271-3. Epub 2012 Dec 27. J Mammary Gland Biol Neoplasia. 2013. PMID: 23269521 Free PMC article. Review.
References
-
- Boyer P. D. (1997) Annu. Rev. Biochem. 66, 717–749 - PubMed
-
- Mitchell P. (1961) Nature 191, 144–148 - PubMed
-
- Abrahams J. P., Leslie A. G., Lutter R., Walker J. E. (1994) Nature 370, 621–628 - PubMed
-
- Yoshida M., Muneyuki E., Hisabori T. (2001) Nat. Rev. Mol. Cell Biol. 2, 669–677 - PubMed
-
- Kucharczyk R., Zick M., Bietenhader M., Rak M., Couplan E., Blondel M., Caubet S. D., di Rago J. P. (2009) Biochim. Biophys. Acta 1793, 186–199 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

