Postischemic deactivation of cardiac aldose reductase: role of glutathione S-transferase P and glutaredoxin in regeneration of reduced thiols from sulfenic acids
- PMID: 20538586
- PMCID: PMC2924019
- DOI: 10.1074/jbc.M110.146423
Postischemic deactivation of cardiac aldose reductase: role of glutathione S-transferase P and glutaredoxin in regeneration of reduced thiols from sulfenic acids
Abstract
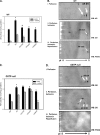
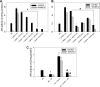
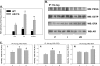
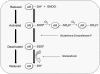
Aldose reductase (AR) is a multifunctional enzyme that catalyzes the reduction of glucose and lipid peroxidation-derived aldehydes. During myocardial ischemia, the activity of AR is increased due to the oxidation of its cysteine residues to sulfenic acids. It is not known, however, whether the activated, sulfenic form of the protein (AR-SOH) is converted back to its reduced, unactivated state (AR-SH). We report here that in perfused mouse hearts activation of AR during 15 min of global ischemia is completely reversed by 30 min of reperfusion. During reperfusion, AR-SOH was converted to a mixed disulfide (AR-SSG). Deactivation of AR and the appearance of AR-SSG during reperfusion were delayed in hearts of mice lacking glutathione S-transferase P (GSTP). In vitro, GSTP accelerated glutathiolation and inactivation of AR-SOH. Reduction of AR-SSG to AR-SH was facilitated by glutaredoxin (GRX). Ischemic activation of AR was increased in GRX-null hearts but was attenuated in the hearts of cardiospecific GRX transgenic mice. Incubation of AR-SSG with GRX led to the regeneration of the reduced form of the enzyme. In ischemic cardiospecific AR transgenic hearts, AR was co-immunoprecipitated with GSTP, whereas in reperfused hearts, the association of AR with GRX was increased. These findings suggest that upon reperfusion of the ischemic heart AR-SOH is converted to AR-SSG via GSTP-assisted glutathiolation. AR-SSG is then reduced by GRX to AR-SH. Sequential catalysis by GSTP and GRX may be a general redox switching mechanism that regulates the reduction of protein sulfenic acids to cysteines.
Figures



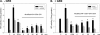

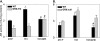
Similar articles
-
Redox activation of aldose reductase in the ischemic heart.J Biol Chem. 2006 Jun 2;281(22):15110-20. doi: 10.1074/jbc.M600837200. Epub 2006 Mar 27. J Biol Chem. 2006. PMID: 16567803
-
Mechanism of 1-Cys type methionine sulfoxide reductase A regeneration by glutaredoxin.Biochem Biophys Res Commun. 2015 Feb 20;457(4):567-71. doi: 10.1016/j.bbrc.2015.01.025. Epub 2015 Jan 16. Biochem Biophys Res Commun. 2015. PMID: 25600814
-
Aldose reductase decreases endoplasmic reticulum stress in ischemic hearts.Chem Biol Interact. 2009 Mar 16;178(1-3):242-9. doi: 10.1016/j.cbi.2008.10.055. Epub 2008 Nov 11. Chem Biol Interact. 2009. PMID: 19041636 Free PMC article.
-
Mechanisms of reversible protein glutathionylation in redox signaling and oxidative stress.Curr Opin Pharmacol. 2007 Aug;7(4):381-91. doi: 10.1016/j.coph.2007.06.003. Epub 2007 Jul 26. Curr Opin Pharmacol. 2007. PMID: 17662654 Review.
-
Protein-sulfenic acids: diverse roles for an unlikely player in enzyme catalysis and redox regulation.Biochemistry. 1999 Nov 23;38(47):15407-16. doi: 10.1021/bi992025k. Biochemistry. 1999. PMID: 10569923 Review.
Cited by
-
S-Glutathionylation of hepatic and visceral adipose proteins decreases in obese rats.Obesity (Silver Spring). 2013 Feb;21(2):297-305. doi: 10.1002/oby.20002. Obesity (Silver Spring). 2013. PMID: 23404913 Free PMC article.
-
Measurement of Reactive Oxygen Species, Reactive Nitrogen Species, and Redox-Dependent Signaling in the Cardiovascular System: A Scientific Statement From the American Heart Association.Circ Res. 2016 Aug 19;119(5):e39-75. doi: 10.1161/RES.0000000000000110. Epub 2016 Jul 14. Circ Res. 2016. PMID: 27418630 Free PMC article. Review.
-
Protein S-glutathiolation: redox-sensitive regulation of protein function.J Mol Cell Cardiol. 2012 Mar;52(3):559-67. doi: 10.1016/j.yjmcc.2011.07.009. Epub 2011 Jul 20. J Mol Cell Cardiol. 2012. PMID: 21784079 Free PMC article. Review.
-
Redox Regulation via Glutaredoxin-1 and Protein S-Glutathionylation.Antioxid Redox Signal. 2020 Apr 1;32(10):677-700. doi: 10.1089/ars.2019.7963. Epub 2020 Jan 23. Antioxid Redox Signal. 2020. PMID: 31813265 Free PMC article. Review.
-
Biological chemistry and functionality of protein sulfenic acids and related thiol modifications.Free Radic Res. 2016;50(2):172-94. doi: 10.3109/10715762.2015.1090571. Epub 2015 Nov 11. Free Radic Res. 2016. PMID: 26340608 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

