Role of zinc in human islet amyloid polypeptide aggregation
- PMID: 20536124
- PMCID: PMC2904811
- DOI: 10.1021/ja1007867
Role of zinc in human islet amyloid polypeptide aggregation
Abstract
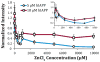
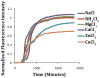
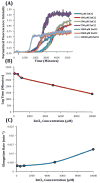
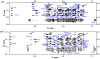
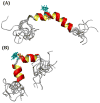
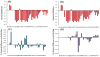
Human Islet Amyloid Polypeptide (hIAPP) is a highly amyloidogenic protein found in islet cells of patients with type II diabetes. Because hIAPP is highly toxic to beta-cells under certain conditions, it has been proposed that hIAPP is linked to the loss of beta-cells and insulin secretion in type II diabetics. One of the interesting questions surrounding this peptide is how the toxic and aggregation prone hIAPP peptide can be maintained in a safe state at the high concentrations that are found in the secretory granule where it is stored. We show here zinc, which is found at millimolar concentrations in the secretory granule, significantly inhibits hIAPP amyloid fibrillogenesis at concentrations similar to those found in the extracellular environment. Zinc has a dual effect on hIAPP fibrillogenesis: it increases the lag-time for fiber formation and decreases the rate of addition of hIAPP to existing fibers at lower concentrations, while having the opposite effect at higher concentrations. Experiments at an acidic pH which partially neutralizes the change in charge upon zinc binding show inhibition is largely due to an electrostatic effect at His18. High-resolution structures of hIAPP determined from NMR experiments confirm zinc binding to His18 and indicate zinc induces localized disruption of the secondary structure of IAPP in the vicinity of His18 of a putative helical intermediate of IAPP. The inhibition of the formation of aggregated and toxic forms of hIAPP by zinc provides a possible mechanism between the recent discovery of linkage between deleterious mutations in the SLC30A8 zinc transporter, which transports zinc into the secretory granule, and type II diabetes.
Figures
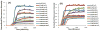




Similar articles
-
A two-site mechanism for the inhibition of IAPP amyloidogenesis by zinc.J Mol Biol. 2011 Jul 8;410(2):294-306. doi: 10.1016/j.jmb.2011.05.015. Epub 2011 May 17. J Mol Biol. 2011. PMID: 21616080 Free PMC article.
-
Investigation of the effects of two major secretory granules components, insulin and zinc, on human-IAPP amyloid aggregation and membrane damage.Chem Phys Lipids. 2021 Jul;237:105083. doi: 10.1016/j.chemphyslip.2021.105083. Epub 2021 Apr 19. Chem Phys Lipids. 2021. PMID: 33887213
-
Pancreatic beta-cell granule peptides form heteromolecular complexes which inhibit islet amyloid polypeptide fibril formation.Biochem J. 2004 Feb 1;377(Pt 3):709-16. doi: 10.1042/BJ20030852. Biochem J. 2004. PMID: 14565847 Free PMC article.
-
Islet amyloid and type 2 diabetes: from molecular misfolding to islet pathophysiology.Biochim Biophys Acta. 2001 Nov 29;1537(3):179-203. doi: 10.1016/s0925-4439(01)00078-3. Biochim Biophys Acta. 2001. PMID: 11731221 Review.
-
The β-cell assassin: IAPP cytotoxicity.J Mol Endocrinol. 2017 Oct;59(3):R121-R140. doi: 10.1530/JME-17-0105. Epub 2017 Aug 15. J Mol Endocrinol. 2017. PMID: 28811318 Review.
Cited by
-
Structure and assembly mechanisms of toxic human islet amyloid polypeptide oligomers associated with copper.Chem Sci. 2016 Aug 1;7(8):5398-5406. doi: 10.1039/c6sc00153j. Epub 2016 May 16. Chem Sci. 2016. PMID: 30155193 Free PMC article.
-
α-CGRP disrupts amylin fibrillization and regulates insulin secretion: implications on diabetes and migraine.Chem Sci. 2021 Mar 24;12(16):5853-5864. doi: 10.1039/d1sc01167g. Chem Sci. 2021. PMID: 34168810 Free PMC article.
-
Inside the Insulin Secretory Granule.Metabolites. 2021 Aug 5;11(8):515. doi: 10.3390/metabo11080515. Metabolites. 2021. PMID: 34436456 Free PMC article. Review.
-
Exploring the central region of amylin and its analogs aggregation: the influence of metal ions and residue substitutions.Front Chem. 2024 Jul 8;12:1419019. doi: 10.3389/fchem.2024.1419019. eCollection 2024. Front Chem. 2024. PMID: 39072260 Free PMC article.
-
An Iridium(III) Complex as a Photoactivatable Tool for Oxidation of Amyloidogenic Peptides with Subsequent Modulation of Peptide Aggregation.Chemistry. 2017 Jan 31;23(7):1645-1653. doi: 10.1002/chem.201604751. Epub 2017 Jan 3. Chemistry. 2017. PMID: 27862428 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

