Role of the Kaposi's sarcoma-associated herpesvirus K15 SH3 binding site in inflammatory signaling and B-cell activation
- PMID: 20534855
- PMCID: PMC2916533
- DOI: 10.1128/JVI.01696-09
Role of the Kaposi's sarcoma-associated herpesvirus K15 SH3 binding site in inflammatory signaling and B-cell activation
Abstract
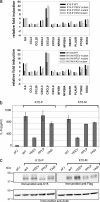
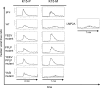
The Kaposi's sarcoma-associated herpesvirus (KSHV) contains several open reading frames (ORFs) that encode proteins capable of initiating and modulating cellular signaling pathways. Among them is ORF K15, encoding a 12-transmembrane-spanning protein with a cytoplasmic C-terminal domain. Through conserved binding motifs, such as Src homology 2 (SH2) and SH3 binding sites, K15 interacts with cellular proteins, activates the NF-kappaB, MEK/Erk, and Jun N-terminal protein kinase (JNK) pathways, and induces the expression of several inflammatory and angiogenic genes. In this study, we investigated the role of an SH3 domain binding site centered on a PPLP motif in K15. We screened libraries of cellular SH3 domains to identify signaling molecules interacting with the KSHV PPLP motif. We found its affinities for two Src kinase family members, Lyn and Hck, to exceed those of other viral proteins. While the SH2 binding motif YEEV is essential for the inflammatory response induced by KSHV K15, recruitment of Lyn and Hck to the K15 PPLP motif seems to be dispensable for this inflammatory response. However, the PPLP motif is essential for the decrease in B-cell receptor-mediated signaling induced by K15, as measured by calcium mobilization assays.
Figures



Similar articles
-
Multi-transmembrane protein K15 of Kaposi's sarcoma-associated herpesvirus targets Lyn kinase in the membrane raft and induces NFAT/AP1 activities.Exp Mol Med. 2008 Oct 31;40(5):565-73. doi: 10.3858/emm.2008.40.5.565. Exp Mol Med. 2008. PMID: 18985015 Free PMC article.
-
Activation of mitogen-activated protein kinase and NF-kappaB pathways by a Kaposi's sarcoma-associated herpesvirus K15 membrane protein.J Virol. 2003 Sep;77(17):9346-58. doi: 10.1128/jvi.77.17.9346-9358.2003. J Virol. 2003. PMID: 12915550 Free PMC article.
-
The K15 protein of Kaposi's sarcoma-associated herpesvirus recruits the endocytic regulator intersectin 2 through a selective SH3 domain interaction.Biochemistry. 2007 Sep 4;46(35):9874-85. doi: 10.1021/bi700357s. Epub 2007 Aug 15. Biochemistry. 2007. PMID: 17696407
-
KSHV non-structural membrane proteins involved in the activation of intracellular signaling pathways and the pathogenesis of Kaposi's sarcoma.Curr Opin Virol. 2016 Oct;20:11-19. doi: 10.1016/j.coviro.2016.07.008. Epub 2016 Aug 9. Curr Opin Virol. 2016. PMID: 27518127 Review.
-
Molecular piracy of Kaposi's sarcoma associated herpesvirus.Cytokine Growth Factor Rev. 2001 Jun-Sep;12(2-3):245-57. doi: 10.1016/s1359-6101(00)00029-0. Cytokine Growth Factor Rev. 2001. PMID: 11325605 Review.
Cited by
-
SH3 domain-mediated recruitment of host cell amphiphysins by alphavirus nsP3 promotes viral RNA replication.PLoS Pathog. 2011 Nov;7(11):e1002383. doi: 10.1371/journal.ppat.1002383. Epub 2011 Nov 17. PLoS Pathog. 2011. PMID: 22114558 Free PMC article.
-
Modulation of Cellular CpG DNA Methylation by Kaposi's Sarcoma-Associated Herpesvirus.J Virol. 2018 Jul 31;92(16):e00008-18. doi: 10.1128/JVI.00008-18. Print 2018 Aug 15. J Virol. 2018. PMID: 29899086 Free PMC article.
-
K15 Protein of Kaposi's Sarcoma Herpesviruses Increases Endothelial Cell Proliferation and Migration through Store-Operated Calcium Entry.Viruses. 2018 May 24;10(6):282. doi: 10.3390/v10060282. Viruses. 2018. PMID: 29795033 Free PMC article.
-
Activation of NF-κB by the Kaposi's sarcoma-associated herpesvirus K15 protein involves recruitment of the NF-κB-inducing kinase, IκB kinases, and phosphorylation of p65.J Virol. 2014 Nov;88(22):13161-72. doi: 10.1128/JVI.01766-14. Epub 2014 Sep 3. J Virol. 2014. PMID: 25187543 Free PMC article.
-
KSHV infection of B-cell lymphoma using a modified KSHV BAC36 and coculturing system.J Microbiol. 2012 Apr;50(2):285-92. doi: 10.1007/s12275-012-1495-9. Epub 2012 Apr 27. J Microbiol. 2012. PMID: 22538658
References
-
- Boshoff, C., T. F. Schulz, M. M. Kennedy, A. K. Graham, C. Fisher, A. Thomas, J. O. McGee, R. A. Weiss, and J. J. O'Leary. 1995. Kaposi's sarcoma-associated herpesvirus infects endothelial and spindle cells. Nat. Med. 1:1274-1278. - PubMed
-
- Brinkmann, M. M., and T. F. Schulz. 2006. Regulation of intracellular signalling by the terminal membrane proteins of members of the Gammaherpesvirinae. J. Gen. Virol. 87:1047-1074. - PubMed
-
- Cesarman, E., Y. Chang, P. S. Moore, J. W. Said, and D. M. Knowles. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186-1191. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

