Frizzled-5, a receptor for the synaptic organizer Wnt7a, regulates activity-mediated synaptogenesis
- PMID: 20530549
- PMCID: PMC2882138
- DOI: 10.1242/dev.046722
Frizzled-5, a receptor for the synaptic organizer Wnt7a, regulates activity-mediated synaptogenesis
Abstract
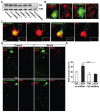
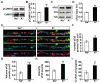
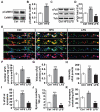
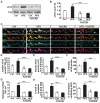
Wnt proteins play a crucial role in several aspects of neuronal circuit formation. Wnts can signal through different receptors including Frizzled, Ryk and Ror2. In the hippocampus, Wnt7a stimulates the formation of synapses; however, its receptor remains poorly characterized. Here, we demonstrate that Frizzled-5 (Fz5) is expressed during the peak of synaptogenesis in the mouse hippocampus. Fz5 is present in synaptosomes and colocalizes with the pre- and postsynaptic markers vGlut1 and PSD-95. Expression of Fz5 during early stages of synaptogenesis increases the number of presynaptic sites in hippocampal neurons. Conversely, Fz5 knockdown or the soluble Fz5-CRD domain (Fz5CRD), which binds to Wnt7a, block the ability of Wnt7a to stimulate synaptogenesis. Increased neuronal activity induced by K+ depolarization or by high-frequency stimulation (HFS), known to induce synapse formation, raises the levels of Fz5 at the cell surface. Importantly, both stimuli increase the localization of Fz5 at synapses, an effect that is blocked by Wnt antagonists or Fz5CRD. Conversely, low-frequency stimulation, which reduces the number of synapses, decreases the levels of surface Fz5 and the percentage of synapses containing the receptor. Interestingly, Fz5CRD abolishes HFS-induced synapse formation. Our results indicate that Fz5 mediates the synaptogenic effect of Wnt7a and that its localization to synapses is regulated by neuronal activity, a process that depends on endogenous Wnts. These findings support a model where neuronal activity and Wnts increase the responsiveness of neurons to Wnt signalling by recruiting Fz5 receptor at synaptic sites.
Figures
Similar articles
-
S-acylation of the Wnt receptor Frizzled-5 by zDHHC5 controls its cellular localization and synaptogenic activity in the rodent hippocampus.Dev Cell. 2023 Oct 23;58(20):2063-2079.e9. doi: 10.1016/j.devcel.2023.07.012. Epub 2023 Aug 8. Dev Cell. 2023. PMID: 37557176
-
Role of the Wnt receptor Frizzled-1 in presynaptic differentiation and function.Neural Dev. 2009 Nov 2;4:41. doi: 10.1186/1749-8104-4-41. Neural Dev. 2009. PMID: 19883499 Free PMC article.
-
Wnts acting through canonical and noncanonical signaling pathways exert opposite effects on hippocampal synapse formation.Neural Dev. 2008 Nov 5;3:32. doi: 10.1186/1749-8104-3-32. Neural Dev. 2008. PMID: 18986540 Free PMC article.
-
Wnt signaling during synaptic development and plasticity.Curr Opin Neurobiol. 2011 Feb;21(1):151-9. doi: 10.1016/j.conb.2010.12.002. Epub 2011 Jan 14. Curr Opin Neurobiol. 2011. PMID: 21239163 Free PMC article. Review.
-
Dual roles for Wnt signalling during the formation of the vertebrate neuromuscular junction.Acta Physiol (Oxf). 2012 Jan;204(1):128-36. doi: 10.1111/j.1748-1716.2011.02295.x. Epub 2011 May 7. Acta Physiol (Oxf). 2012. PMID: 21554559 Review.
Cited by
-
Neurotrophin and Wnt signaling cooperatively regulate dendritic spine formation.Mol Cell Neurosci. 2013 Sep;56:115-27. doi: 10.1016/j.mcn.2013.04.006. Epub 2013 Apr 30. Mol Cell Neurosci. 2013. PMID: 23639831 Free PMC article.
-
SPARCL1 Promotes Excitatory But Not Inhibitory Synapse Formation and Function Independent of Neurexins and Neuroligins.J Neurosci. 2020 Oct 14;40(42):8088-8102. doi: 10.1523/JNEUROSCI.0454-20.2020. Epub 2020 Sep 24. J Neurosci. 2020. PMID: 32973045 Free PMC article.
-
Wnt signaling in synaptogenesis of Alzheimer's disease.Ibrain. 2023 Sep 6;9(3):316-325. doi: 10.1002/ibra.12130. eCollection 2023 Fall. Ibrain. 2023. PMID: 37786762 Free PMC article. Review.
-
Hippocampal Wnt Signaling: Memory Regulation and Hormone Interactions.Neuroscientist. 2016 Jun;22(3):278-94. doi: 10.1177/1073858415574728. Epub 2015 Feb 25. Neuroscientist. 2016. PMID: 25717070 Free PMC article. Review.
-
Deficient Wnt Signaling and Synaptic Vulnerability in Alzheimer's Disease: Emerging Roles for the LRP6 Receptor.Front Synaptic Neurosci. 2018 Oct 30;10:38. doi: 10.3389/fnsyn.2018.00038. eCollection 2018. Front Synaptic Neurosci. 2018. PMID: 30425633 Free PMC article. Review.
References
-
- Angers S., Moon R. T. (2009). Proximal events in Wnt signal transduction. Nat. Rev. Mol. Cell Biol. 10, 468-477 - PubMed
-
- Antonova I., Arancio O., Trillat A. C., Wang H. G., Zablow L., Udo H., Kandel E. R., Hawkins R. D. (2001). Rapid increase in clusters of presynaptic proteins at onset of long-lasting potentiation. Science 294, 1547-1550 - PubMed
-
- Attwell D., Laughlin S. B. (2001). An energy budget for signaling in the grey matter of the brain. J. Cereb. Blood Flow Metab. 21, 1133-1145 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

