Randomized phase III trial of ixabepilone plus capecitabine versus capecitabine in patients with metastatic breast cancer previously treated with an anthracycline and a taxane
- PMID: 20530276
- PMCID: PMC2903325
- DOI: 10.1200/JCO.2009.24.4244
Randomized phase III trial of ixabepilone plus capecitabine versus capecitabine in patients with metastatic breast cancer previously treated with an anthracycline and a taxane
Abstract
Purpose: We sought to determine whether the combination of ixabepilone plus capecitabine improved overall survival (OS) compared with capecitabine alone in patients with metastatic breast cancer (MBC) previously treated with anthracyclines and taxanes.
Patients and methods: A total of 1,221 patients with MBC previously treated with anthracycline and taxanes were randomly assigned to ixabepilone (40 mg/m(2) intravenously on day 1) plus capecitabine (2,000 mg/m(2) orally on days 1 through 14) or capecitabine alone (2,500 mg/m(2) on the same schedule) given every 21 days. The trial was powered to detect a 20% reduction in the hazard ratio (HR) for death.
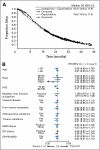
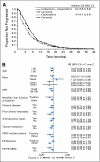
Results: There was no significant difference in OS between the combination and capecitabine monotherapy arm, the primary end point (median, 16.4 v 15.6 months; HR = 0.9; 95% CI, 078 to 1.03; P = .1162). The arms were well balanced with the exception of a higher prevalence of impaired performance status (Karnofsky performance status 70% to 80%) in the combination arm (32% v 25%). In a secondary Cox regression analysis adjusted for performance status and other prognostic factors, OS was improved for the combination (HR = 0.85; 95% CI, 0.75 to 0.98; P = .0231). In 79% of patients with measurable disease, the combination significantly improved progression-free survival (PFS; median, 6.2 v 4.2 months; HR = 0.79; P = .0005) and response rate (43% v 29%; P < .0001). Grade 3 to 4 neuropathy occurred in 24% treated with the combination, but was reversible.
Conclusion: This study confirmed a previous trial demonstrating improved PFS and response for the ixabepilone-capecitabine combination compared with capecitabine alone, although this did not result in improved survival.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Similar articles
-
Analysis of overall survival from a phase III study of ixabepilone plus capecitabine versus capecitabine in patients with MBC resistant to anthracyclines and taxanes.Breast Cancer Res Treat. 2010 Jul;122(2):409-18. doi: 10.1007/s10549-010-0901-4. Epub 2010 May 8. Breast Cancer Res Treat. 2010. PMID: 20454927 Clinical Trial.
-
Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment.J Clin Oncol. 2007 Nov 20;25(33):5210-7. doi: 10.1200/JCO.2007.12.6557. Epub 2007 Oct 29. J Clin Oncol. 2007. PMID: 17968020 Clinical Trial.
-
Efficacy and safety of ixabepilone plus capecitabine in elderly patients with anthracycline- and taxane-pretreated metastatic breast cancer.J Geriatr Oncol. 2013 Oct;4(4):346-52. doi: 10.1016/j.jgo.2013.07.006. Epub 2013 Aug 23. J Geriatr Oncol. 2013. PMID: 24472478 Clinical Trial.
-
Ixabepilone plus capecitabine for breast cancer patients with an early metastatic relapse after adjuvant chemotherapy: two clinical trials.Clin Breast Cancer. 2010 Oct 1;10(5):352-8. doi: 10.3816/CBC.2010.n.046. Clin Breast Cancer. 2010. PMID: 20920979 Review.
-
The optimal therapeutic use of ixabepilone in patients with locally advanced or metastatic breast cancer.J Oncol Pharm Pract. 2009 Jun;15(2):95-106. doi: 10.1177/1078155208100095. Epub 2009 Jan 26. J Oncol Pharm Pract. 2009. PMID: 19171553 Review.
Cited by
-
An Easy and Efficient Strategy for the Enhancement of Epothilone Production Mediated by TALE-TF and CRISPR/dcas9 Systems in Sorangium cellulosum.Front Bioeng Biotechnol. 2019 Nov 26;7:334. doi: 10.3389/fbioe.2019.00334. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 32039165 Free PMC article.
-
Ixabepilone: Overview of Effectiveness, Safety, and Tolerability in Metastatic Breast Cancer.Front Oncol. 2021 Jul 6;11:617874. doi: 10.3389/fonc.2021.617874. eCollection 2021. Front Oncol. 2021. PMID: 34295806 Free PMC article. Review.
-
Chemotherapy in Patients with Anthracycline- and Taxane-Pretreated Metastatic Breast Cancer: An Overview.Curr Breast Cancer Rep. 2013 Mar 1;5(1):42-50. doi: 10.1007/s12609-012-0097-1. Curr Breast Cancer Rep. 2013. PMID: 23440080 Free PMC article.
-
Which patients with metastatic breast cancer benefit from subsequent lines of treatment? An update for clinicians.Ther Adv Med Oncol. 2013 Nov;5(6):334-50. doi: 10.1177/1758834013508197. Ther Adv Med Oncol. 2013. PMID: 24179488 Free PMC article.
-
Overall survival as the outcome for randomized clinical trials with effective subsequent therapies.J Clin Oncol. 2011 Jun 10;29(17):2439-42. doi: 10.1200/JCO.2011.34.6056. Epub 2011 May 9. J Clin Oncol. 2011. PMID: 21555691 Free PMC article. Review.
References
-
- Surveillance Epidemiology and End Results: Estimated new cancer cases and deaths for 2006. http://seer.cancer.gov/csr/1975_2003/results_resulsingle/resulsect_resul....
-
- Blum JL, Jones SE, Buzdar AU, et al. Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol. 1999;17:485–493. - PubMed
-
- Fumoleau P, Largillier R, Clippe C, et al. Multicentre, phase II study evaluating capecitabine monotherapy in patients with anthracycline- and taxane-pretreated metastatic breast cancer. Eur J Cancer. 2004;40:536–542. - PubMed
-
- Lee JJ, Swain SM. Development of novel chemotherapeutic agents to evade the mechanisms of multidrug resistance (MDR) Semin Oncol. 2005;32:S22–S26. - PubMed
-
- Fumoleau P, Coudert B, Isambert N, et al. Novel tubulin-targeting agents: Anticancer activity and pharmacologic profile of epothilones and related analogues. Ann Oncol. 2007;18(suppl 5):v9–v15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

