TDP-43 mediates degeneration in a novel Drosophila model of disease caused by mutations in VCP/p97
- PMID: 20519548
- PMCID: PMC2890254
- DOI: 10.1523/JNEUROSCI.5894-09.2010
TDP-43 mediates degeneration in a novel Drosophila model of disease caused by mutations in VCP/p97
Abstract
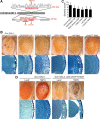
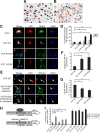
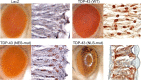
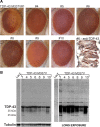
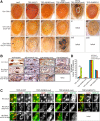
Inclusion body myopathy associated with Paget's disease of bone and frontotemporal dementia (IBMPFD) is a dominantly inherited degenerative disorder caused by mutations in the valosin-containing protein (VCP7) gene. VCP (p97 in mouse, TER94 in Drosophila melanogaster, and CDC48 in Saccharomyces cerevisiae) is a highly conserved AAA(+) (ATPases associated with multiple cellular activities) ATPase that regulates a wide array of cellular processes. The mechanism of IBMPFD pathogenesis is unknown. To elucidate the pathogenic mechanism, we developed and characterized a Drosophila model of IBMPFD (mutant-VCP-related degeneration). Based on genetic screening of this model, we identified three RNA-binding proteins that dominantly suppressed degeneration; one of these was TBPH, the Drosophila homolog of TAR (trans-activating response region) DNA-binding protein 43 (TDP-43). Here we demonstrate that VCP and TDP-43 interact genetically and that disease-causing mutations in VCP lead to redistribution of TDP-43 to the cytoplasm in vitro and in vivo, replicating the major pathology observed in IBMPFD and other TDP-43 proteinopathies. We also demonstrate that TDP-43 redistribution from the nucleus to the cytoplasm is sufficient to induce cytotoxicity. Furthermore, we determined that a pathogenic mutation in TDP-43 promotes redistribution to the cytoplasm and enhances the genetic interaction with VCP. Together, our results show that degeneration associated with VCP mutations is mediated in part by toxic gain of function of TDP-43 in the cytoplasm. We suggest that these findings are likely relevant to the pathogenic mechanism of a broad array of TDP-43 proteinopathies, including frontotemporal lobar degeneration and amyotrophic lateral sclerosis.
Figures

Similar articles
-
Transgenic mice expressing mutant forms VCP/p97 recapitulate the full spectrum of IBMPFD including degeneration in muscle, brain and bone.Hum Mol Genet. 2010 May 1;19(9):1741-55. doi: 10.1093/hmg/ddq050. Epub 2010 Feb 10. Hum Mol Genet. 2010. PMID: 20147319
-
Pathogenic VCP/TER94 alleles are dominant actives and contribute to neurodegeneration by altering cellular ATP level in a Drosophila IBMPFD model.PLoS Genet. 2011 Feb 3;7(2):e1001288. doi: 10.1371/journal.pgen.1001288. PLoS Genet. 2011. PMID: 21304887 Free PMC article.
-
The multiple faces of valosin-containing protein-associated diseases: inclusion body myopathy with Paget's disease of bone, frontotemporal dementia, and amyotrophic lateral sclerosis.J Mol Neurosci. 2011 Nov;45(3):522-31. doi: 10.1007/s12031-011-9627-y. Epub 2011 Sep 3. J Mol Neurosci. 2011. PMID: 21892620 Review.
-
Phenotypic variability in three families with valosin-containing protein mutation.Eur J Neurol. 2013 Feb;20(2):251-8. doi: 10.1111/j.1468-1331.2012.03831.x. Epub 2012 Aug 20. Eur J Neurol. 2013. PMID: 22900631 Free PMC article.
-
Inclusion body myopathy, Paget's disease of the bone and fronto-temporal dementia: a disorder of autophagy.Hum Mol Genet. 2010 Apr 15;19(R1):R38-45. doi: 10.1093/hmg/ddq157. Epub 2010 Apr 21. Hum Mol Genet. 2010. PMID: 20410287 Free PMC article. Review.
Cited by
-
The Role of Small Heat Shock Proteins in Protein Misfolding Associated Motoneuron Diseases.Int J Mol Sci. 2022 Oct 4;23(19):11759. doi: 10.3390/ijms231911759. Int J Mol Sci. 2022. PMID: 36233058 Free PMC article. Review.
-
Aggrephagy: selective disposal of protein aggregates by macroautophagy.Int J Cell Biol. 2012;2012:736905. doi: 10.1155/2012/736905. Epub 2012 Mar 22. Int J Cell Biol. 2012. PMID: 22518139 Free PMC article.
-
Amyloid fibril proteomics of AD brains reveals modifiers of aggregation and toxicity.Mol Neurodegener. 2023 Sep 14;18(1):61. doi: 10.1186/s13024-023-00654-z. Mol Neurodegener. 2023. PMID: 37710351 Free PMC article.
-
The complexities of p97 function in health and disease.Mol Biosyst. 2011 Mar;7(3):700-10. doi: 10.1039/c0mb00176g. Epub 2010 Dec 14. Mol Biosyst. 2011. PMID: 21152665 Free PMC article. Review.
-
TDP-43 suppresses CGG repeat-induced neurotoxicity through interactions with HnRNP A2/B1.Hum Mol Genet. 2014 Oct 1;23(19):5036-51. doi: 10.1093/hmg/ddu216. Epub 2014 May 8. Hum Mol Genet. 2014. PMID: 24920338 Free PMC article.
References
-
- Acharya KK, Govind CK, Shore AN, Stoler MH, Reddi PP. cis-requirement for the maintenance of round spermatid-specific transcription. Dev Biol. 2006;295:781–790. - PubMed
-
- Ayala YM, Zago P, D'Ambrogio A, Xu YF, Petrucelli L, Buratti E, Baralle FE. Structural determinants of the cellular localization and shuttling of TDP-43. J Cell Sci. 2008;121:3778–3785. - PubMed
-
- Brand A, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. - PubMed
-
- Colombrita C, Zennaro E, Fallini C, Weber M, Sommacal A, Buratti E, Silani V, Ratti A. TDP-43 is recruited to stress granules in conditions of oxidative insult. J Neurochem. 2009;111:1051–1061. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
