Pim1 promotes human prostate cancer cell tumorigenicity and c-MYC transcriptional activity
- PMID: 20515470
- PMCID: PMC2886047
- DOI: 10.1186/1471-2407-10-248
Pim1 promotes human prostate cancer cell tumorigenicity and c-MYC transcriptional activity
Abstract
Background: The serine/threonine kinase PIM1 has been implicated as an oncogene in various human cancers including lymphomas, gastric, colorectal and prostate carcinomas. In mouse models, Pim1 is known to cooperate with c-Myc to promote tumorigenicity. However, there has been limited analysis of the tumorigenic potential of Pim1 overexpression in benign and malignant human prostate cancer cells in vivo.
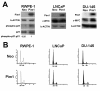
Methods: We overexpressed Pim1 in three human prostate cell lines representing different disease stages including benign (RWPE1), androgen-dependent cancer (LNCaP) and androgen-independent cancer (DU145). We then analyzed in vitro and in vivo tumorigenicity as well as the effect of Pim1 overexpression on c-MYC transcriptional activity by reporter assays and gene expression profiling using an inducible MYC-ER system. To validate that Pim1 induces tumorigenicity and target gene expression by modulating c-MYC transcriptional activity, we inhibited c-MYC using a small molecule inhibitor (10058-F4) or RNA interference.
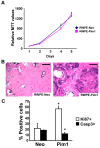
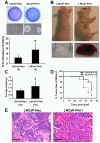
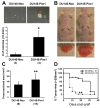
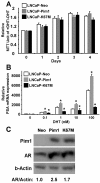
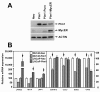
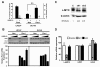
Results: Overexpression of Pim1 alone was not sufficient to convert the benign RWPE1 cell to malignancy although it enhanced their proliferation rates when grown as xenografts in vivo. However, Pim1 expression enhanced the in vitro and in vivo tumorigenic potentials of the human prostate cancer cell lines LNCaP and DU145. Reporter assays revealed increased c-MYC transcriptional activity in Pim1-expressing cells and mRNA expression profiling demonstrated that a large fraction of c-MYC target genes were also regulated by Pim1 expression. The c-MYC inhibitor 10058-F4 suppressed the tumorigenicity of Pim1-expressing prostate cancer cells. Interestingly, 10058-F4 treatment also led to a reduction of Pim1 protein but not mRNA. Knocking-down c-MYC using short hairpin RNA reversed the effects of Pim1 on Pim1/MYC target genes.
Conclusion: Our results suggest an in vivo role of Pim1 in promoting prostate tumorigenesis although it displayed distinct oncogenic activities depending on the disease stage of the cell line. Pim1 promotes tumorigenicity at least in part by enhancing c-MYC transcriptional activity. We also made the novel discovery that treatment of cells with the c-MYC inhibitor 10058-F4 leads to a reduction in Pim1 protein levels.
Figures
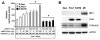
Similar articles
-
Pim1 kinase is required to maintain tumorigenicity in MYC-expressing prostate cancer cells.Oncogene. 2012 Apr 5;31(14):1794-803. doi: 10.1038/onc.2011.371. Epub 2011 Aug 22. Oncogene. 2012. PMID: 21860423 Free PMC article.
-
PIM1 overexpression in T-cell lymphomas protects tumor cells from apoptosis and confers doxorubicin resistance by upregulating c-myc expression.Acta Biochim Biophys Sin (Shanghai). 2018 Aug 1;50(8):800-806. doi: 10.1093/abbs/gmy076. Acta Biochim Biophys Sin (Shanghai). 2018. PMID: 30020405
-
Pim1 regulates androgen-dependent survival signaling in prostate cancer cells.Urol Int. 2010;84(2):212-20. doi: 10.1159/000277601. Epub 2010 Mar 4. Urol Int. 2010. PMID: 20215828
-
PIM1 kinase as a target in prostate cancer: roles in tumorigenesis, castration resistance, and docetaxel resistance.Curr Cancer Drug Targets. 2014;14(2):105-14. doi: 10.2174/1568009613666131126113854. Curr Cancer Drug Targets. 2014. PMID: 24274399 Review.
-
Why target PIM1 for cancer diagnosis and treatment?Future Oncol. 2010 Sep;6(9):1461-78. doi: 10.2217/fon.10.106. Future Oncol. 2010. PMID: 20919829 Free PMC article. Review.
Cited by
-
Co-Targeting PIM Kinase and PI3K/mTOR in NSCLC.Cancers (Basel). 2021 Apr 29;13(9):2139. doi: 10.3390/cancers13092139. Cancers (Basel). 2021. PMID: 33946744 Free PMC article.
-
Troglitazone suppresses c-Myc levels in human prostate cancer cells via a PPARγ-independent mechanism.Cancer Biol Ther. 2011 Jun 15;11(12):1046-58. doi: 10.4161/cbt.11.12.15709. Epub 2011 Jun 15. Cancer Biol Ther. 2011. PMID: 21525782 Free PMC article.
-
RNAi screen identifies a synthetic lethal interaction between PIM1 overexpression and PLK1 inhibition.Clin Cancer Res. 2014 Jun 15;20(12):3211-21. doi: 10.1158/1078-0432.CCR-13-3116. Epub 2014 Apr 25. Clin Cancer Res. 2014. PMID: 24771642 Free PMC article.
-
The Multifaceted Role of Aldehyde Dehydrogenases in Prostate Cancer Stem Cells.Cancers (Basel). 2021 Sep 20;13(18):4703. doi: 10.3390/cancers13184703. Cancers (Basel). 2021. PMID: 34572930 Free PMC article. Review.
-
PIM kinase (and Akt) biology and signaling in tumors.Pharmacol Ther. 2015 Jul;151:41-9. doi: 10.1016/j.pharmthera.2015.03.001. Epub 2015 Mar 5. Pharmacol Ther. 2015. PMID: 25749412 Free PMC article. Review.
References
-
- Mochizuki T, Kitanaka C, Noguchi K, Muramatsu T, Asai A, Kuchino Y. Physical and functional interactions between Pim-1 kinase and Cdc25A phosphatase. Implications for the Pim-1-mediated activation of the c-Myc signaling pathway. J Biol Chem. 1999;274(26):18659–18666. doi: 10.1074/jbc.274.26.18659. - DOI - PubMed
-
- Bachmann M, Hennemann H, Xing PX, Hoffmann I, Moroy T. The oncogenic serine/threonine kinase Pim-1 phosphorylates and inhibits the activity of Cdc25C-associated kinase 1 (C-TAK1): a novel role for Pim-1 at the G2/M cell cycle checkpoint. J Biol Chem. 2004;279(46):48319–48328. doi: 10.1074/jbc.M404440200. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

