Lethal dissemination of H5N1 influenza virus is associated with dysregulation of inflammation and lipoxin signaling in a mouse model of infection
- PMID: 20504916
- PMCID: PMC2897611
- DOI: 10.1128/JVI.00553-10
Lethal dissemination of H5N1 influenza virus is associated with dysregulation of inflammation and lipoxin signaling in a mouse model of infection
Abstract
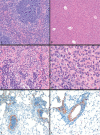
Periodic outbreaks of highly pathogenic avian H5N1 influenza viruses and the current H1N1 pandemic highlight the need for a more detailed understanding of influenza virus pathogenesis. To investigate the host transcriptional response induced by pathogenic influenza viruses, we used a functional-genomics approach to compare gene expression profiles in lungs from 129S6/SvEv mice infected with either the fully reconstructed H1N1 1918 pandemic virus (1918) or the highly pathogenic avian H5N1 virus Vietnam/1203/04 (VN/1203). Although the viruses reached similar titers in the lung and caused lethal infections, the mean time of death was 6 days for VN/1203-infected animals and 9 days for mice infected with the 1918 virus. VN/1203-infected animals also exhibited an earlier and more potent inflammatory response. This response included induction of genes encoding components of the inflammasome. VN/1203 was also able to disseminate to multiple organs, including the brain, which correlated with changes in the expression of genes associated with hematological functions and lipoxin biogenesis and signaling. Both viruses elicited expression of type I interferon (IFN)-regulated genes in wild-type mice and to a lesser extent in mice lacking the type I IFN receptor, suggesting alternative or redundant pathways for IFN signaling. Our findings suggest that VN/1203 is more pathogenic in mice as a consequence of several factors, including the early and sustained induction of the inflammatory response, the additive or synergistic effects of upregulated components of the immune response, and inhibition of lipoxin-mediated anti-inflammatory responses, which correlated with the ability of VN/1203 to disseminate to extrapulmonary organs.
Figures


Similar articles
-
Lethal influenza virus infection in macaques is associated with early dysregulation of inflammatory related genes.PLoS Pathog. 2009 Oct;5(10):e1000604. doi: 10.1371/journal.ppat.1000604. Epub 2009 Oct 2. PLoS Pathog. 2009. PMID: 19798428 Free PMC article.
-
CLEC5A-Mediated Enhancement of the Inflammatory Response in Myeloid Cells Contributes to Influenza Virus Pathogenicity In Vivo.J Virol. 2016 Dec 16;91(1):e01813-16. doi: 10.1128/JVI.01813-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27795434 Free PMC article.
-
Highly pathogenic avian influenza A H5N1 and pandemic H1N1 virus infections have different phenotypes in Toll-like receptor 3 knockout mice.J Gen Virol. 2014 Sep;95(Pt 9):1870-1879. doi: 10.1099/vir.0.066258-0. Epub 2014 May 30. J Gen Virol. 2014. PMID: 24878639 Free PMC article.
-
Innate immune responses to influenza A H5N1: friend or foe?Trends Immunol. 2009 Dec;30(12):574-84. doi: 10.1016/j.it.2009.09.004. Epub 2009 Oct 26. Trends Immunol. 2009. PMID: 19864182 Free PMC article. Review.
-
The brain-liver cholinergic anti-inflammatory pathway and viral infections.Bioelectron Med. 2023 Dec 20;9(1):29. doi: 10.1186/s42234-023-00132-3. Bioelectron Med. 2023. PMID: 38115148 Free PMC article. Review.
Cited by
-
Systems biology from virus to humans.J Anal Sci Technol. 2015;6(1):3. doi: 10.1186/s40543-015-0047-4. Epub 2015 Feb 5. J Anal Sci Technol. 2015. PMID: 26269748 Free PMC article. Review.
-
Temporal dynamics of host molecular responses differentiate symptomatic and asymptomatic influenza a infection.PLoS Genet. 2011 Aug;7(8):e1002234. doi: 10.1371/journal.pgen.1002234. Epub 2011 Aug 25. PLoS Genet. 2011. PMID: 21901105 Free PMC article.
-
Cytokine Release Syndrome in COVID-19 Patients, A New Scenario for an Old Concern: The Fragile Balance between Infections and Autoimmunity.Int J Mol Sci. 2020 May 8;21(9):3330. doi: 10.3390/ijms21093330. Int J Mol Sci. 2020. PMID: 32397174 Free PMC article. Review.
-
Quantitative genetics in the study of virus-induced disease.Adv Virus Res. 2014;88:193-225. doi: 10.1016/B978-0-12-800098-4.00004-0. Adv Virus Res. 2014. PMID: 24373313 Free PMC article. Review.
-
Immunomodulation of periodontitis with SPMs.Front Oral Health. 2023 Oct 20;4:1288722. doi: 10.3389/froh.2023.1288722. eCollection 2023. Front Oral Health. 2023. PMID: 37927821 Free PMC article. Review.
References
-
- Agarwal, P. P., S. Cinti, and E. A. Kazerooni. 2009. Chest radiographic and CT findings in novel swine-origin influenza A (H1N1) virus (S-OIV) infection. Am. J. Roentgenol. 193:1488-1493. - PubMed
-
- Ank, N., M. B. Iversen, C. Bartholdy, P. Staeheli, R. Hartmann, U. B. Jensen, F. Dagnaes-Hansen, A. R. Thomsen, Z. Chen, H. Haugen, K. Klucher, and S. R. Paludan. 2008. An important role for type III interferon (IFN-lambda/IL-28) in TLR-induced antiviral activity. J. Immunol. 180:2474-2485. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

