Targeting tissue factor on tumour cells and angiogenic vascular endothelial cells by factor VII-targeted verteporfin photodynamic therapy for breast cancer in vitro and in vivo in mice
- PMID: 20504328
- PMCID: PMC2882923
- DOI: 10.1186/1471-2407-10-235
Targeting tissue factor on tumour cells and angiogenic vascular endothelial cells by factor VII-targeted verteporfin photodynamic therapy for breast cancer in vitro and in vivo in mice
Abstract
Background: The objective of this study was to develop a ligand-targeted photodynamic therapy (tPDT) by conjugating factor VII (fVII) protein with photosensitiser verteporfin in order to overcome the poor selectivity and enhance the effect of non-targeted PDT (ntPDT) for cancer. fVII is a natural ligand for receptor tissue factor (TF) with high affinity and specificity. The reason for targeting receptor TF for the development of tPDT is that TF is a common but specific target on angiogenic tumour vascular endothelial cells (VEC) and many types of tumour cells, including solid tumours and leukaemia.
Methods: Murine factor VII protein (mfVII) containing a mutation (Lys341Ala) was covalently conjugated via a cross linker EDC with Veterporfin (VP) that was extracted from liposomal Visudyne, and then free VP was separated by Sephadex G50 spin columns. fVII-tPDT using mfVII-VP conjugate, compared to ntPDT, was tested in vitro for the killing of breast cancer cells and VEGF-stimulated VEC and in vivo for inhibiting the tumour growth of breast tumours in a mouse xenograft model.
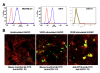
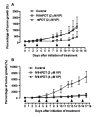
Results: We showed that: (i) fVII protein could be conjugated with VP without affecting its binding activity; (ii) fVII-tPDT could selectively kill TF-expressing breast cancer cells and VEGF-stimulated angiogenic HUVECs but had no side effects on non-TF expressing unstimulated HUVEC, CHO-K1 and 293 cells; (iii) fVII targeting enhanced the effect of VP PDT by three to four fold; (iii) fVII-tPDT induced significantly stronger levels of apoptosis and necrosis than ntPDT; and (iv) fVII-tPDT had a significantly stronger effect on inhibiting breast tumour growth in mice than ntPDT.
Conclusions: We conclude that the fVII-targeted VP PDT that we report here is a novel and effective therapeutic with improved selectivity for the treatment of breast cancer. Since TF is expressed on many types of cancer cells including leukaemic cells and selectively on angiogenic tumour VECs, fVII-tPDT could have broad therapeutic applications for other solid cancers and leukaemia.
Figures

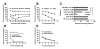


Similar articles
-
Effective treatment of chemoresistant breast cancer in vitro and in vivo by a factor VII-targeted photodynamic therapy.Br J Cancer. 2011 Apr 26;104(9):1401-9. doi: 10.1038/bjc.2011.88. Epub 2011 Mar 22. Br J Cancer. 2011. PMID: 21427724 Free PMC article.
-
Selective and effective killing of angiogenic vascular endothelial cells and cancer cells by targeting tissue factor using a factor VII-targeted photodynamic therapy for breast cancer.Breast Cancer Res Treat. 2011 Apr;126(3):589-600. doi: 10.1007/s10549-010-0957-1. Epub 2010 Jun 1. Breast Cancer Res Treat. 2011. PMID: 20514515 Free PMC article.
-
Effective treatment of human lung cancer by targeting tissue factor with a factor VII-targeted photodynamic therapy.Curr Cancer Drug Targets. 2011 Nov;11(9):1069-81. doi: 10.2174/156800911798073023. Curr Cancer Drug Targets. 2011. PMID: 21933104
-
Your bleeding heart: lessons from low tissue factor expression in mice.Trends Pharmacol Sci. 2003 Jun;24(6):269-72. doi: 10.1016/S0165-6147(03)00121-4. Trends Pharmacol Sci. 2003. PMID: 12823950 Review.
-
Tissue factor in the myocardium: evidence of roles in haemostasis and inflammation.Dis Markers. 2004;20(6):353-8. doi: 10.1155/2004/963402. Dis Markers. 2004. PMID: 15665396 Free PMC article. Review.
Cited by
-
Neoadjuvant vascular-targeted photodynamic therapy improves survival and reduces recurrence and progression in a mouse model of urothelial cancer.Sci Rep. 2021 Mar 1;11(1):4842. doi: 10.1038/s41598-021-84184-y. Sci Rep. 2021. PMID: 33649388 Free PMC article.
-
Recombinant epidermal growth factor-like domain-1 from coagulation factor VII functionalized iron oxide nanoparticles for targeted glioma magnetic resonance imaging.Int J Nanomedicine. 2016 Oct 6;11:5099-5108. doi: 10.2147/IJN.S116980. eCollection 2016. Int J Nanomedicine. 2016. PMID: 27785017 Free PMC article.
-
Apoptosis of HeLa cells induced by a new targeting photosensitizer-based PDT via a mitochondrial pathway and ER stress.Onco Targets Ther. 2015 Apr 7;8:703-11. doi: 10.2147/OTT.S76370. eCollection 2015. Onco Targets Ther. 2015. PMID: 25897245 Free PMC article.
-
Tissue factor as a new target for CAR-NK cell immunotherapy of triple-negative breast cancer.Sci Rep. 2020 Feb 18;10(1):2815. doi: 10.1038/s41598-020-59736-3. Sci Rep. 2020. PMID: 32071339 Free PMC article.
-
Targeting Tissue Factor to Tumor Vasculature to Induce Tumor Infarction.Cancers (Basel). 2021 Jun 7;13(11):2841. doi: 10.3390/cancers13112841. Cancers (Basel). 2021. PMID: 34200318 Free PMC article. Review.
References
-
- Hu Z, Sun Y, Garen A. Targeting tumor vasculature endothelial cells and tumor cells for immunotherapy of human melanoma in a mouse xenograft model. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(14):8161–8166. doi: 10.1073/pnas.96.14.8161. - DOI - PMC - PubMed
-
- Hu Z, Garen A. Targeting tissue factor on tumor vascular endothelial cells and tumor cells for immunotherapy in mouse models of prostatic cancer. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(21):12180–12185. doi: 10.1073/pnas.201420298. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

