Persistent growth of a human plasma-derived hepatitis C virus genotype 1b isolate in cell culture
- PMID: 20502631
- PMCID: PMC2873922
- DOI: 10.1371/journal.ppat.1000910
Persistent growth of a human plasma-derived hepatitis C virus genotype 1b isolate in cell culture
Abstract
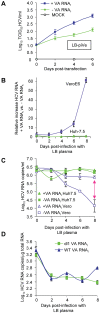
HCV (hepatitis C virus) research, including therapeutics and vaccine development, has been hampered by the lack of suitable tissue culture models. Development of cell culture systems for the growth of the most drug-resistant HCV genotype (1b) as well as natural isolates has remained a challenge. Transfection of cultured cells with adenovirus-associated RNA(I) (VA RNA(I)), a known interferon (IFN) antagonist and inhibitor of dsRNA-mediated antiviral pathways, enhanced the growth of plasma-derived HCV genotype 1b. Furthermore, persistent viral growth was achieved after passaging through IFN-alpha/beta-deficient VeroE6 cells for 2 years. Persistently infected cells were maintained in culture for an additional 4 years, and the virus rescued from these cells induced strong cytopathic effect (CPE). Using a CPE-based assay, we measured inhibition of viral production by anti-HCV specific inhibitors, including 2'-C-Methyl-D-Adenosine, demonstrating its utility for the evaluation of HCV antivirals. This virus constitutes a novel tool for the study of one of the most relevant strains of HCV, genotype 1b, which will now be available for HCV life cycle research and useful for the development of new therapeutics.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
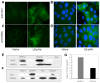

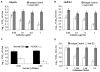
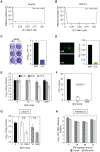
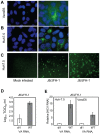
Similar articles
-
The non-structural 5A protein of hepatitis C virus exhibits genotypic differences in interferon antagonism.J Hepatol. 2008 Dec;49(6):899-907. doi: 10.1016/j.jhep.2008.06.030. Epub 2008 Sep 16. J Hepatol. 2008. PMID: 18842320
-
Analysis of genotypes and amino acid residues 2209 to 2248 of the NS5A region of hepatitis C virus in relation to the response to interferon-beta therapy.Hepatology. 1997 Mar;25(3):750-3. doi: 10.1002/hep.510250343. Hepatology. 1997. PMID: 9049230
-
Current status and future development of infectious cell-culture models for the major genotypes of hepatitis C virus: Essential tools in testing of antivirals and emerging vaccine strategies.Antiviral Res. 2018 Oct;158:264-287. doi: 10.1016/j.antiviral.2018.07.014. Epub 2018 Jul 27. Antiviral Res. 2018. PMID: 30059723 Review.
-
Mutations in the nonstructural 5A gene of European hepatitis C virus isolates and response to interferon alfa.Hepatology. 1997 Mar;25(3):740-4. doi: 10.1002/hep.510250341. Hepatology. 1997. PMID: 9049228
-
Cutting the gordian knot-development and biological relevance of hepatitis C virus cell culture systems.Adv Virus Res. 2008;71:51-133. doi: 10.1016/S0065-3527(08)00002-X. Adv Virus Res. 2008. PMID: 18585527 Review.
Cited by
-
A new model to produce infectious hepatitis C virus without the replication requirement.PLoS Pathog. 2011 Apr;7(4):e1001333. doi: 10.1371/journal.ppat.1001333. Epub 2011 Apr 14. PLoS Pathog. 2011. PMID: 21533214 Free PMC article. Review.
-
Cell culture replication of a genotype 1b hepatitis C virus isolate cloned from a patient who underwent liver transplantation.PLoS One. 2011;6(8):e23587. doi: 10.1371/journal.pone.0023587. Epub 2011 Aug 24. PLoS One. 2011. PMID: 21887279 Free PMC article.
-
HCV-Mediated Apoptosis of Hepatocytes in Culture and Viral Pathogenesis.PLoS One. 2016 Jun 9;11(6):e0155708. doi: 10.1371/journal.pone.0155708. eCollection 2016. PLoS One. 2016. PMID: 27280444 Free PMC article.
-
Productive hepatitis C virus infection of stem cell-derived hepatocytes reveals a critical transition to viral permissiveness during differentiation.PLoS Pathog. 2012;8(4):e1002617. doi: 10.1371/journal.ppat.1002617. Epub 2012 Apr 5. PLoS Pathog. 2012. PMID: 22496645 Free PMC article.
-
Highly efficient full-length hepatitis C virus genotype 1 (strain TN) infectious culture system.Proc Natl Acad Sci U S A. 2012 Nov 27;109(48):19757-62. doi: 10.1073/pnas.1218260109. Epub 2012 Nov 14. Proc Natl Acad Sci U S A. 2012. PMID: 23151512 Free PMC article.
References
-
- Simmonds P, Bukh J, Combet C, Deleage G, Enomoto N, et al. Consensus proposals for a unified system of nomenclature of hepatitis C virus genotypes. Hepatology. 2005;42:962–973. - PubMed
-
- Lindenbach BD, Evans MJ, Syder AJ, Wolk B, Tellinghuisen TL, et al. Complete replication of hepatitis C virus in cell culture. Science. 2005;309:623–626. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

