Microbiota innate stimulation is a prerequisite for T cell spontaneous proliferation and induction of experimental colitis
- PMID: 20498021
- PMCID: PMC2882839
- DOI: 10.1084/jem.20092253
Microbiota innate stimulation is a prerequisite for T cell spontaneous proliferation and induction of experimental colitis
Erratum in
- J Exp Med. 2010 Jul 5;207(7):1569
Abstract
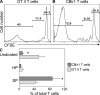
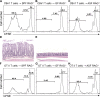
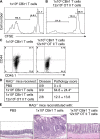
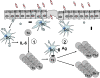
Little is known about how the microbiota regulates T cell proliferation and whether spontaneous T cell proliferation is involved in the pathogenesis of inflammatory bowel disease. In this study, we show that stimulation of innate pathways by microbiota-derived ligands and antigen-specific T cell stimulation are both required for intestinal inflammation. Microbiota-derived ligands promoted spontaneous T cell proliferation by activating dendritic cells (DCs) to produce IL-6 via Myd88, as shown by the spontaneous proliferation of T cells adoptively transferred into specific pathogen-free (SPF) RAG-/- mice, but not in germfree RAG-/- mice. Reconstitution of germfree RAG-/- mice with cecal bacterial lysate-pulsed DCs, but not with IL-6-/- or Myd88-/- DCs, restored spontaneous T cell proliferation. CBir1 TCR transgenic (CBir1 Tg) T cells, which are specific for an immunodominant microbiota antigen, induced colitis in SPF RAG-/- mice. Blocking the spontaneous proliferation of CBir1 Tg T cells by co-transferring bulk OT II CD4+ T cells abrogated colitis development. Although transferred OT II T cells underwent spontaneous proliferation in RAG-/- mice, the recipients failed to develop colitis because of the lack of cognate antigen in the intestinal lumen. Collectively, our data demonstrate that induction of colitis requires both spontaneous proliferation of T cells driven by microbiota-derived innate signals and antigen-specific T cell proliferation.
Figures



Similar articles
-
Th17 cells induce colitis and promote Th1 cell responses through IL-17 induction of innate IL-12 and IL-23 production.J Immunol. 2011 Jun 1;186(11):6313-8. doi: 10.4049/jimmunol.1001454. Epub 2011 Apr 29. J Immunol. 2011. PMID: 21531892 Free PMC article.
-
Microbiota: dual-faceted player in experimental colitis.Gut Microbes. 2010 Nov-Dec;1(6):388-91. doi: 10.4161/gmic.1.6.13727. Gut Microbes. 2010. PMID: 21468221 Free PMC article. Review.
-
Interleukin-12 converts Foxp3+ regulatory T cells to interferon-γ-producing Foxp3+ T cells that inhibit colitis.Gastroenterology. 2011 Jun;140(7):2031-43. doi: 10.1053/j.gastro.2011.03.009. Epub 2011 Mar 17. Gastroenterology. 2011. PMID: 21419767 Free PMC article.
-
Wiskott-Aldrich syndrome protein deficiency in innate immune cells leads to mucosal immune dysregulation and colitis in mice.Gastroenterology. 2012 Sep;143(3):719-729.e2. doi: 10.1053/j.gastro.2012.06.008. Epub 2012 Jun 15. Gastroenterology. 2012. PMID: 22710191 Free PMC article.
-
Diet Modifies Colonic Microbiota and CD4+ T-Cell Repertoire to Induce Flares of Colitis in Mice With Myeloid-Cell Expression of Interleukin 23.Gastroenterology. 2018 Oct;155(4):1177-1191.e16. doi: 10.1053/j.gastro.2018.06.034. Epub 2018 Jun 15. Gastroenterology. 2018. PMID: 29909020 Free PMC article.
Cited by
-
Type I IFNs regulate effector and regulatory T cell accumulation and anti-inflammatory cytokine production during T cell-mediated colitis.J Immunol. 2013 Sep 1;191(5):2771-9. doi: 10.4049/jimmunol.1301093. Epub 2013 Aug 2. J Immunol. 2013. PMID: 23913971 Free PMC article.
-
Commensal Bacteria-Specific CD4+ T Cell Responses in Health and Disease.Front Immunol. 2018 Nov 20;9:2667. doi: 10.3389/fimmu.2018.02667. eCollection 2018. Front Immunol. 2018. PMID: 30524431 Free PMC article. Review.
-
Spontaneous Proliferation of CD4+ T Cells in RAG-Deficient Hosts Promotes Antigen-Independent but IL-2-Dependent Strong Proliferative Response of Naïve CD8+ T Cells.Front Immunol. 2018 Aug 23;9:1907. doi: 10.3389/fimmu.2018.01907. eCollection 2018. Front Immunol. 2018. PMID: 30190718 Free PMC article.
-
A gut feeling about murine syngeneic GVHD.Chimerism. 2011 Apr;2(2):58-60. doi: 10.4161/chim.2.2.16783. Chimerism. 2011. PMID: 21912721 Free PMC article.
-
Th17 cells induce colitis and promote Th1 cell responses through IL-17 induction of innate IL-12 and IL-23 production.J Immunol. 2011 Jun 1;186(11):6313-8. doi: 10.4049/jimmunol.1001454. Epub 2011 Apr 29. J Immunol. 2011. PMID: 21531892 Free PMC article.
References
-
- Bourgeois C., Stockinger B. 2006. CD25+CD4+ regulatory T cells and memory T cells prevent lymphopenia-induced proliferation of naive T cells in transient states of lymphopenia. J. Immunol. 177:4558–4566 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

