Regulation of O-glycosylation through Golgi-to-ER relocation of initiation enzymes
- PMID: 20498016
- PMCID: PMC2878949
- DOI: 10.1083/jcb.201003055
Regulation of O-glycosylation through Golgi-to-ER relocation of initiation enzymes
Abstract
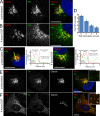
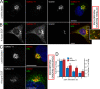
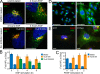
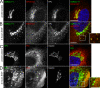
After growth factor stimulation, kinases are activated to regulate multiple aspects of cell physiology. Activated Src is present on Golgi membranes, but its function here remains unclear. We find that Src regulates mucin-type protein O-glycosylation through redistribution of the initiating enzymes, polypeptide N-acetylgalactosaminyl transferases (GalNac-Ts), from the Golgi to the ER. Redistribution occurs after stimulation with EGF or PDGF in a Src-dependent manner and in cells with constitutively elevated Src activity. All GalNac-T family enzymes tested are affected, whereas multiple other glycosylation enzymes are not displaced from the Golgi. Upon Src activation, the COP-I coat is also redistributed in punctate structures that colocalize with GalNac-Ts and a dominant-negative Arf1 isoform, Arf1(Q71L), efficiently blocks GalNac-T redistribution, indicating that Src activates a COP-I-dependent trafficking event. Finally, Src activation increases O-glycosylation initiation as seen by lectin staining and metabolic labeling. We propose that growth factor stimulation regulates O-glycosylation initiation in a Src-dependent fashion by GalNac-T redistribution to the ER.
Figures
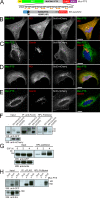
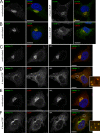
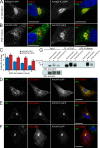

Comment in
-
Comment on "The GalNAc-T Activation Pathway (GALA) is not a general mechanism for regulating mucin-type O-glycosylation".PLoS One. 2017 Jul 18;12(7):e0180005. doi: 10.1371/journal.pone.0180005. eCollection 2017. PLoS One. 2017. PMID: 28719645 Free PMC article. No abstract available.
Similar articles
-
The GalNAc-T Activation Pathway (GALA) is not a general mechanism for regulating mucin-type O-glycosylation.PLoS One. 2017 Jul 18;12(7):e0179241. doi: 10.1371/journal.pone.0179241. eCollection 2017. PLoS One. 2017. PMID: 28719662 Free PMC article.
-
Localization of three human polypeptide GalNAc-transferases in HeLa cells suggests initiation of O-linked glycosylation throughout the Golgi apparatus.J Cell Sci. 1998 Jan;111 ( Pt 1):45-60. doi: 10.1242/jcs.111.1.45. J Cell Sci. 1998. PMID: 9394011
-
Glycosylation of α-dystroglycan: O-mannosylation influences the subsequent addition of GalNAc by UDP-GalNAc polypeptide N-acetylgalactosaminyltransferases.J Biol Chem. 2012 Jun 15;287(25):20967-74. doi: 10.1074/jbc.M112.370387. Epub 2012 May 1. J Biol Chem. 2012. PMID: 22549772 Free PMC article.
-
Location, location, location: new insights into O-GalNAc protein glycosylation.Trends Cell Biol. 2011 Mar;21(3):149-58. doi: 10.1016/j.tcb.2010.11.004. Epub 2010 Dec 8. Trends Cell Biol. 2011. PMID: 21145746 Review.
-
Recent structural and mechanistic insights into protein O-GalNAc glycosylation.Biochem Soc Trans. 2016 Feb;44(1):61-7. doi: 10.1042/BST20150178. Biochem Soc Trans. 2016. PMID: 26862189 Review.
Cited by
-
A non-enzymatic function of Golgi glycosyltransferases: mediation of Golgi fragmentation by interaction with non-muscle myosin IIA.Glycobiology. 2013 Jun;23(6):690-708. doi: 10.1093/glycob/cwt009. Epub 2013 Feb 7. Glycobiology. 2013. PMID: 23396488 Free PMC article.
-
Vertebrate protein glycosylation: diversity, synthesis and function.Nat Rev Mol Cell Biol. 2012 Jun 22;13(7):448-62. doi: 10.1038/nrm3383. Nat Rev Mol Cell Biol. 2012. PMID: 22722607 Free PMC article. Review.
-
Intestinal epithelial glycosylation in homeostasis and gut microbiota interactions in IBD.Nat Rev Gastroenterol Hepatol. 2020 Oct;17(10):597-617. doi: 10.1038/s41575-020-0331-7. Epub 2020 Jul 24. Nat Rev Gastroenterol Hepatol. 2020. PMID: 32710014 Free PMC article. Review.
-
Endogenous and Exogenous Regulatory Signaling in the Secretory Pathway: Role of Golgi Signaling Molecules in Cancer.Front Cell Dev Biol. 2022 Mar 23;10:833663. doi: 10.3389/fcell.2022.833663. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35399533 Free PMC article. Review.
-
Polypeptide-GalNAc-T6 expression predicts better overall survival in patients with colon cancer.Oncol Lett. 2018 Jul;16(1):225-234. doi: 10.3892/ol.2018.8686. Epub 2018 May 10. Oncol Lett. 2018. PMID: 29928405 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

