JAK2 V617F impairs hematopoietic stem cell function in a conditional knock-in mouse model of JAK2 V617F-positive essential thrombocythemia
- PMID: 20489053
- PMCID: PMC3145111
- DOI: 10.1182/blood-2009-12-259747
JAK2 V617F impairs hematopoietic stem cell function in a conditional knock-in mouse model of JAK2 V617F-positive essential thrombocythemia
Abstract
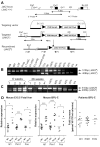
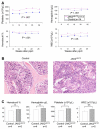
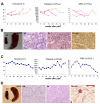
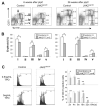
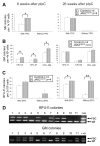
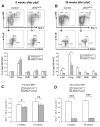
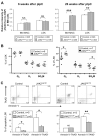
The JAK2 V617F mutation is found in most patients with a myeloproliferative neoplasm and is sufficient to produce a myeloproliferative phenotype in murine retroviral transplantation or transgenic models. However, several lines of evidence suggest that disease phenotype is influenced by the level of mutant JAK2 signaling, and we have therefore generated a conditional knock-in mouse in which a human JAK2 V617F is expressed under the control of the mouse Jak2 locus. Human and murine Jak2 transcripts are expressed at similar levels, and mice develop modest increases in hemoglobin and platelet levels reminiscent of human JAK2 V617F-positive essential thrombocythemia. The phenotype is transplantable and accompanied by increased terminal erythroid and megakaryocyte differentiation together with increased numbers of clonogenic progenitors, including erythropoietin-independent erythroid colonies. Unexpectedly, JAK2(V617F) mice develop reduced numbers of lineage(-)Sca-1(+)c-Kit(+) cells, which exhibit increased DNA damage, reduced apoptosis, and reduced cell cycling. Moreover, competitive bone marrow transplantation studies demonstrated impaired hematopoietic stem cell function in JAK2(V617F) mice. These results suggest that the chronicity of human myeloproliferative neoplasms may reflect a balance between impaired hematopoietic stem cell function and the accumulation of additional mutations.
Figures
Comment in
-
JAK2 impairs stem cell function?Blood. 2010 Sep 2;116(9):1392-3. doi: 10.1182/blood-2010-06-287318. Blood. 2010. PMID: 20813904 No abstract available.
Similar articles
-
A role for reactive oxygen species in JAK2 V617F myeloproliferative neoplasm progression.Leukemia. 2013 Nov;27(11):2187-95. doi: 10.1038/leu.2013.102. Epub 2013 Apr 5. Leukemia. 2013. PMID: 23558526
-
Loss of Stat1 decreases megakaryopoiesis and favors erythropoiesis in a JAK2-V617F-driven mouse model of MPNs.Blood. 2014 Jun 19;123(25):3943-50. doi: 10.1182/blood-2013-07-514208. Epub 2014 May 12. Blood. 2014. PMID: 24820309
-
Deregulation of apoptosis-related genes is associated with PRV1 overexpression and JAK2 V617F allele burden in Essential Thrombocythemia and Myelofibrosis.J Hematol Oncol. 2012 Feb 2;5:2. doi: 10.1186/1756-8722-5-2. J Hematol Oncol. 2012. PMID: 22300941 Free PMC article.
-
[Myeloproliferative diseases caused by JAK2 mutation].Rinsho Byori. 2009 Apr;57(4):357-64. Rinsho Byori. 2009. PMID: 19489438 Review. Japanese.
-
Current diagnostic criteria for the chronic myeloproliferative disorders (MPD) essential thrombocythemia (ET), polycythemia vera (PV) and chronic idiopathic myelofibrosis (CIMF).Pathol Biol (Paris). 2007 Mar;55(2):92-104. doi: 10.1016/j.patbio.2006.06.002. Epub 2006 Aug 21. Pathol Biol (Paris). 2007. PMID: 16919893 Review.
Cited by
-
Cooperativity of imprinted genes inactivated by acquired chromosome 20q deletions.J Clin Invest. 2013 May;123(5):2169-82. doi: 10.1172/JCI66113. Epub 2013 Apr 1. J Clin Invest. 2013. PMID: 23543057 Free PMC article.
-
Distinct roles for long-term hematopoietic stem cells and erythroid precursor cells in a murine model of Jak2V617F-mediated polycythemia vera.Blood. 2012 Jul 5;120(1):166-72. doi: 10.1182/blood-2012-01-402396. Epub 2012 May 24. Blood. 2012. PMID: 22627765 Free PMC article.
-
Effect of mutation order on myeloproliferative neoplasms.N Engl J Med. 2015 Feb 12;372(7):601-612. doi: 10.1056/NEJMoa1412098. N Engl J Med. 2015. PMID: 25671252 Free PMC article.
-
Disease burden at the progenitor level is a feature of primary myelofibrosis: a multivariable analysis of 164 JAK2 V617F-positive myeloproliferative neoplasm patients.Exp Hematol. 2011 Jan;39(1):95-101. doi: 10.1016/j.exphem.2010.09.008. Epub 2010 Oct 1. Exp Hematol. 2011. PMID: 20888389 Free PMC article.
-
Leukemic Transformation of Myeloproliferative Neoplasms: Therapeutic and Genomic Considerations.Curr Hematol Malig Rep. 2018 Dec;13(6):588-595. doi: 10.1007/s11899-018-0491-5. Curr Hematol Malig Rep. 2018. PMID: 30353413 Review.
References
-
- James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144–1148. - PubMed
-
- Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365(9464):1054–1061. - PubMed
-
- Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7(4):387–397. - PubMed
-
- Kralovics R, Passamonti F, Teo SS, et al. A gain of function mutation in Jak2 is frequently found in patients with myeloproliferative disorders. N Engl J Med. 2005;352(17):1779–1790. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

