Inhibition of pulmonary fibrosis in mice by CXCL10 requires glycosaminoglycan binding and syndecan-4
- PMID: 20484822
- PMCID: PMC2877927
- DOI: 10.1172/JCI38644
Inhibition of pulmonary fibrosis in mice by CXCL10 requires glycosaminoglycan binding and syndecan-4
Abstract
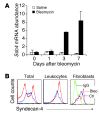
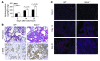
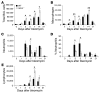
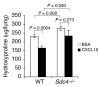
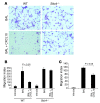
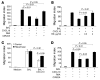
Pulmonary fibrosis is a progressive, dysregulated response to injury culminating in compromised lung function due to excess extracellular matrix production. The heparan sulfate proteoglycan syndecan-4 is important in mediating fibroblast-matrix interactions, but its role in pulmonary fibrosis has not been explored. To investigate this issue, we used intratracheal instillation of bleomycin as a model of acute lung injury and fibrosis. We found that bleomycin treatment increased syndecan-4 expression. Moreover, we observed a marked decrease in neutrophil recruitment and an increase in both myofibroblast recruitment and interstitial fibrosis in bleomycin-treated syndecan-4-null (Sdc4-/-) mice. Subsequently, we identified a direct interaction between CXCL10, an antifibrotic chemokine, and syndecan-4 that inhibited primary lung fibroblast migration during fibrosis; mutation of the heparin-binding domain, but not the CXCR3 domain, of CXCL10 diminished this effect. Similarly, migration of fibroblasts from patients with pulmonary fibrosis was inhibited in the presence of CXCL10 protein defective in CXCR3 binding. Furthermore, administration of recombinant CXCL10 protein inhibited fibrosis in WT mice, but not in Sdc4-/- mice. Collectively, these data suggest that the direct interaction of syndecan-4 and CXCL10 in the lung interstitial compartment serves to inhibit fibroblast recruitment and subsequent fibrosis. Thus, administration of CXCL10 protein defective in CXCR3 binding may represent a novel therapy for pulmonary fibrosis.
Figures


Similar articles
-
Syndecan-4 Inhibits the Development of Pulmonary Fibrosis by Attenuating TGF-β Signaling.Int J Mol Sci. 2019 Oct 9;20(20):4989. doi: 10.3390/ijms20204989. Int J Mol Sci. 2019. PMID: 31600983 Free PMC article.
-
The antifibrotic effects of plasminogen activation occur via prostaglandin E2 synthesis in humans and mice.J Clin Invest. 2010 Jun;120(6):1950-60. doi: 10.1172/JCI38369. Epub 2010 May 24. J Clin Invest. 2010. PMID: 20501949 Free PMC article.
-
Recruited exudative macrophages selectively produce CXCL10 after noninfectious lung injury.Am J Respir Cell Mol Biol. 2011 Oct;45(4):781-8. doi: 10.1165/rcmb.2010-0471OC. Epub 2011 Feb 17. Am J Respir Cell Mol Biol. 2011. PMID: 21330464 Free PMC article.
-
Syndecan-4 regulates early neutrophil migration and pulmonary inflammation in response to lipopolysaccharide.Am J Respir Cell Mol Biol. 2012 Aug;47(2):196-202. doi: 10.1165/rcmb.2011-0294OC. Epub 2012 Mar 15. Am J Respir Cell Mol Biol. 2012. PMID: 22427536 Free PMC article.
-
Megakaryoblastic leukemia-1 is required for the development of bleomycin-induced pulmonary fibrosis.Respir Res. 2015 Mar 27;16(1):45. doi: 10.1186/s12931-015-0206-6. Respir Res. 2015. PMID: 25885656 Free PMC article.
Cited by
-
Reprogramming of profibrotic macrophages for treatment of bleomycin-induced pulmonary fibrosis.EMBO Mol Med. 2020 Aug 7;12(8):e12034. doi: 10.15252/emmm.202012034. Epub 2020 Jun 29. EMBO Mol Med. 2020. PMID: 32597014 Free PMC article.
-
Loss of Twist1 in the Mesenchymal Compartment Promotes Increased Fibrosis in Experimental Lung Injury by Enhanced Expression of CXCL12.J Immunol. 2017 Mar 15;198(6):2269-2285. doi: 10.4049/jimmunol.1600610. Epub 2017 Feb 8. J Immunol. 2017. PMID: 28179498 Free PMC article.
-
Human umbilical cord mesenchymal stromal cells attenuate pulmonary fibrosis via regulatory T cell through interaction with macrophage.Stem Cell Res Ther. 2021 Jul 13;12(1):397. doi: 10.1186/s13287-021-02469-5. Stem Cell Res Ther. 2021. PMID: 34256845 Free PMC article.
-
The neurovascular extracellular matrix in health and disease.Exp Biol Med (Maywood). 2021 Apr;246(7):835-844. doi: 10.1177/1535370220977195. Epub 2020 Dec 10. Exp Biol Med (Maywood). 2021. PMID: 33302738 Free PMC article. Review.
-
Tissue microenvironments define and get reinforced by macrophage phenotypes in homeostasis or during inflammation, repair and fibrosis.J Innate Immun. 2012;4(5-6):463-77. doi: 10.1159/000336717. Epub 2012 Apr 11. J Innate Immun. 2012. PMID: 22507825 Free PMC article. Review.
References
-
- Ramos C, et al. Fibroblasts from idiopathic pulmonary fibrosis and normal lungs differ in growth rate, apoptosis, and tissue inhibitor of metalloproteinases expression. Am J Respir Cell Mol Biol. 2001;24(5):591–598. - PubMed
-
- Selman M, et al. TIMP-1, -2, -3, and -4 in idiopathic pulmonary fibrosis. A prevailing nondegradative lung microenvironment? Am J Physiol Lung Cell Mol Physiol. 2000;279(3):L562–L574. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

