Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease
- PMID: 20484731
- PMCID: PMC3034445
- DOI: 10.1126/scitranslmed.3000632
Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease
Abstract
The pathogenesis of human and simian immunodeficiency viruses is characterized by CD4(+) T cell depletion and chronic T cell activation, leading ultimately to AIDS. CD4(+) T helper (T(H)) cells provide protective immunity and immune regulation through different immune cell functional subsets, including T(H)1, T(H)2, T regulatory (T(reg)), and interleukin-17 (IL-17)-secreting T(H)17 cells. Because IL-17 can enhance host defenses against microbial agents, thus maintaining the integrity of the mucosal barrier, loss of T(H)17 cells may foster microbial translocation and sustained inflammation. Here, we study HIV-seropositive subjects and find that progressive disease is associated with the loss of T(H)17 cells and a reciprocal increase in the fraction of the immunosuppressive T(reg) cells both in peripheral blood and in rectosigmoid biopsies. The loss of T(H)17/T(reg) balance is associated with induction of indoleamine 2,3-dioxygenase 1 (IDO1) by myeloid antigen-presenting dendritic cells and with increased plasma concentration of microbial products. In vitro, the loss of T(H)17/T(reg) balance is mediated directly by the proximal tryptophan catabolite from IDO metabolism, 3-hydroxyanthranilic acid. We postulate that induction of IDO may represent a critical initiating event that results in inversion of the T(H)17/T(reg) balance and in the consequent maintenance of a chronic inflammatory state in progressive HIV disease.
Conflict of interest statement
Figures
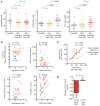
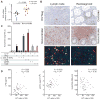
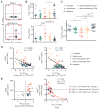

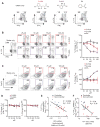
Comment in
-
Insights into therapy: tryptophan oxidation and HIV infection.Sci Transl Med. 2010 May 19;2(32):32ps23. doi: 10.1126/scitranslmed.3001082. Sci Transl Med. 2010. PMID: 20484729
Similar articles
-
Insights into therapy: tryptophan oxidation and HIV infection.Sci Transl Med. 2010 May 19;2(32):32ps23. doi: 10.1126/scitranslmed.3001082. Sci Transl Med. 2010. PMID: 20484729
-
Indoleamine 2,3-dioxygenase-expressing leukemic dendritic cells impair a leukemia-specific immune response by inducing potent T regulatory cells.Haematologica. 2010 Dec;95(12):2022-30. doi: 10.3324/haematol.2010.025924. Epub 2010 Aug 26. Haematologica. 2010. PMID: 20801903 Free PMC article.
-
Clinical Relevance of Kynurenine Pathway in HIV/AIDS: An Immune Checkpoint at the Crossroads of Metabolism and Inflammation.AIDS Rev. 2015 Apr-Jun;17(2):96-106. AIDS Rev. 2015. PMID: 26035167 Review.
-
Interferon-gamma-triggered indoleamine 2,3-dioxygenase competence in human monocyte-derived dendritic cells induces regulatory activity in allogeneic T cells.Blood. 2009 Oct 8;114(15):3235-43. doi: 10.1182/blood-2008-12-195073. Epub 2009 Jul 22. Blood. 2009. PMID: 19625705
-
Indoleamine 2,3-dioxygenase in transplantation.Nephrology (Carlton). 2008 Jun;13(3):204-11. doi: 10.1111/j.1440-1797.2007.00921.x. Epub 2008 Jan 23. Nephrology (Carlton). 2008. PMID: 18221253 Review.
Cited by
-
Immune Characteristics and Immunotherapy of HIV-Associated Lymphoma.Curr Issues Mol Biol. 2024 Sep 10;46(9):9984-9997. doi: 10.3390/cimb46090596. Curr Issues Mol Biol. 2024. PMID: 39329948 Free PMC article. Review.
-
The aryl hydrocarbon receptor pathway: a linking bridge between the gut microbiome and neurodegenerative diseases.Front Cell Neurosci. 2024 Aug 8;18:1433747. doi: 10.3389/fncel.2024.1433747. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39175504 Free PMC article. Review.
-
The Complex Dysregulations of CD4 T Cell Subtypes in HIV Infection.Int J Mol Sci. 2024 Jul 9;25(14):7512. doi: 10.3390/ijms25147512. Int J Mol Sci. 2024. PMID: 39062756 Free PMC article. Review.
-
Tryptophan-Kynurenine Pathway Activation and Cognition in Virally Suppressed Women With HIV.J Acquir Immune Defic Syndr. 2024 Aug 15;96(5):494-500. doi: 10.1097/QAI.0000000000003454. Epub 2024 Jul 9. J Acquir Immune Defic Syndr. 2024. PMID: 38985447 Free PMC article.
-
The combination of Butyricicoccus pullicaecorum and 3-hydroxyanthranilic acid prevents postmenopausal osteoporosis by modulating gut microbiota and Th17/Treg.Eur J Nutr. 2024 Aug;63(5):1945-1959. doi: 10.1007/s00394-024-03400-3. Epub 2024 May 16. Eur J Nutr. 2024. PMID: 38753171 Free PMC article.
References
-
- McCune JM. The dynamics of CD4+ T-cell depletion in HIV disease. Nature. 2001;410:974–979. - PubMed
-
- Valdez H, Lederman MM. Cytokines and cytokine therapies in HIV infection. AIDS Clin Rev. 1997:187–228. - PubMed
-
- Murray MF. Tryptophan depletion and HIV infection: A metabolic link to pathogenesis. Lancet Infect Dis. 2003;3:644–652. - PubMed
-
- Deeks SG, Kitchen CM, Liu L, Guo H, Gascon R, Narváez AB, Hunt P, Martin JN, Kahn JO, Levy J, McGrath MS, Hecht FM. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104:942–947. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI066917/AI/NIAID NIH HHS/United States
- P01 AI071713/AI/NIAID NIH HHS/United States
- K01 AI066917/AI/NIAID NIH HHS/United States
- T32 DK007762/DK/NIDDK NIH HHS/United States
- K24 AI069994/AI/NIAID NIH HHS/United States
- K08 DK083334/DK/NIDDK NIH HHS/United States
- P30 MH59037/MH/NIMH NIH HHS/United States
- P30 AI027763/AI/NIAID NIH HHS/United States
- DPI OD00329/OD/NIH HHS/United States
- R37 AI040312/AI/NIAID NIH HHS/United States
- K08 DK083334-01A1/DK/NIDDK NIH HHS/United States
- P30 AI27763/AI/NIAID NIH HHS/United States
- R37 AI40312/AI/NIAID NIH HHS/United States
- R01 K23 AI065244/AI/NIAID NIH HHS/United States
- P30 AI022763/AI/NIAID NIH HHS/United States
- UL1 RR024131/RR/NCRR NIH HHS/United States
- K23 AI065244/AI/NIAID NIH HHS/United States
- DP1 OD000329/OD/NIH HHS/United States
- AI069994/AI/NIAID NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
- K24 DK060617/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

