Role of the endoplasmic reticulum chaperone BiP, SUN domain proteins, and dynein in altering nuclear morphology during human cytomegalovirus infection
- PMID: 20484513
- PMCID: PMC2898220
- DOI: 10.1128/JVI.00719-10
Role of the endoplasmic reticulum chaperone BiP, SUN domain proteins, and dynein in altering nuclear morphology during human cytomegalovirus infection
Abstract
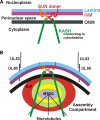
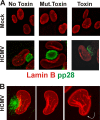
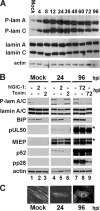
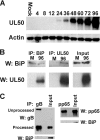
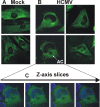
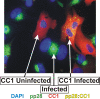
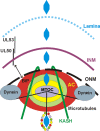
The process of assembly and egress of human cytomegalovirus (HCMV) virions requires significant morphological alterations of the nuclear and cytoplasmic architecture. In the studies presented we show that the nuclear periphery is dramatically altered, especially near the cytoplasmic assembly compartment, where the nuclear lamina is specifically rearranged, the outer nuclear membrane is altered, and the nucleus becomes permeable to large molecules. In addition, the tethering of the inner and outer nuclear membranes is lost during infection due to a decrease in levels of the SUN domain proteins. We previously demonstrated that the endoplasmic reticulum protein BiP functions as a component of the assembly compartment and disruption of BiP causes the loss of assembly compartment integrity. In this study we show that the depletion of BiP, and the loss of assembly compartment integrity, results in the loss of virally induced lamina rearrangement and morphology of the nucleus that is characteristic of HCMV infection. BiP functions in lamina rearrangement through its ability to affect lamin phosphorylation. Depletion of BiP and disruption of the assembly compartment result in the loss of lamin phosphorylation. The dependency of lamin phosphorylation on BiP correlates with an interaction between BiP and UL50. Finally, we confirm previous data (S. V. Indran, M. E. Ballestas, and W. J. Britt, J. Virol. 84:3162-3177, 2010) suggesting an involvement of dynein in assembly compartment formation and extend this observation by showing that when dynein is inhibited, the nuclear morphology characteristic of an HCMV infection is lost. Our data suggest a highly integrated assembly-egress continuum.
Figures



Similar articles
-
The endoplasmic reticulum chaperone BiP/GRP78 is important in the structure and function of the human cytomegalovirus assembly compartment.J Virol. 2009 Nov;83(22):11421-8. doi: 10.1128/JVI.00762-09. Epub 2009 Sep 9. J Virol. 2009. PMID: 19741001 Free PMC article.
-
Human cytomegalovirus specifically controls the levels of the endoplasmic reticulum chaperone BiP/GRP78, which is required for virion assembly.J Virol. 2008 Jan;82(1):31-9. doi: 10.1128/JVI.01881-07. Epub 2007 Oct 17. J Virol. 2008. PMID: 17942541 Free PMC article.
-
Human cytomegalovirus induces the endoplasmic reticulum chaperone BiP through increased transcription and activation of translation by using the BiP internal ribosome entry site.J Virol. 2010 Nov;84(21):11479-86. doi: 10.1128/JVI.01330-10. Epub 2010 Aug 25. J Virol. 2010. PMID: 20739513 Free PMC article.
-
The endoplasmic reticulum (ER) chaperone BiP is a master regulator of ER functions: Getting by with a little help from ERdj friends.J Biol Chem. 2019 Feb 8;294(6):2098-2108. doi: 10.1074/jbc.REV118.002804. Epub 2018 Dec 18. J Biol Chem. 2019. PMID: 30563838 Free PMC article. Review.
-
Nuclear Cytoskeleton in Virus Infection.Int J Mol Sci. 2022 Jan 5;23(1):578. doi: 10.3390/ijms23010578. Int J Mol Sci. 2022. PMID: 35009004 Free PMC article. Review.
Cited by
-
Herpes Simplex Virus Organizes Cytoplasmic Membranes To Form a Viral Assembly Center in Neuronal Cells.J Virol. 2020 Sep 15;94(19):e00900-20. doi: 10.1128/JVI.00900-20. Print 2020 Sep 15. J Virol. 2020. PMID: 32699089 Free PMC article.
-
Cytomegalovirus Late Protein pUL31 Alters Pre-rRNA Expression and Nuclear Organization during Infection.J Virol. 2017 Aug 24;91(18):e00593-17. doi: 10.1128/JVI.00593-17. Print 2017 Sep 15. J Virol. 2017. PMID: 28659485 Free PMC article.
-
Cis and trans acting factors involved in human cytomegalovirus experimental and natural latent infection of CD14 (+) monocytes and CD34 (+) cells.PLoS Pathog. 2013;9(5):e1003366. doi: 10.1371/journal.ppat.1003366. Epub 2013 May 23. PLoS Pathog. 2013. PMID: 23717203 Free PMC article. Clinical Trial.
-
Herpesviruses remodel host membranes for virus egress.Nat Rev Microbiol. 2011 May;9(5):382-94. doi: 10.1038/nrmicro2559. Nat Rev Microbiol. 2011. PMID: 21494278 Review.
-
Nuclear Egress Complexes of HCMV and Other Herpesviruses: Solving the Puzzle of Sequence Coevolution, Conserved Structures and Subfamily-Spanning Binding Properties.Viruses. 2020 Jun 24;12(6):683. doi: 10.3390/v12060683. Viruses. 2020. PMID: 32599939 Free PMC article. Review.
References
-
- Azzeh, M., A. Honigman, A. Taraboulos, A. Rouvinski, and D. G. Wolf. 2006. Structural changes in human cytomegalovirus cytoplasmic assembly sites in the absence of UL97 kinase activity. Virology 354:69-79. - PubMed
-
- Beaudouin, J., D. Gerlich, N. Daigle, R. Eils, and J. Ellenberg. 2002. Nuclear envelope breakdown proceeds by microtubule-induced tearing of the lamina. Cell 108:83-96. - PubMed
-
- Beck, K. A. 2005. Spectrins and the Golgi. Biochim. Biophys. Acta 1744:374-382. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

