Ric-8A and Gi alpha recruit LGN, NuMA, and dynein to the cell cortex to help orient the mitotic spindle
- PMID: 20479129
- PMCID: PMC2897540
- DOI: 10.1128/MCB.00394-10
Ric-8A and Gi alpha recruit LGN, NuMA, and dynein to the cell cortex to help orient the mitotic spindle
Abstract
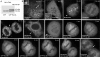
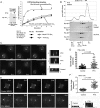
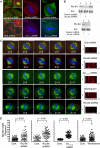
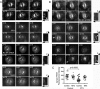
In model organisms, resistance to inhibitors of cholinesterase 8 (Ric-8), a G protein alpha (G alpha) subunit guanine nucleotide exchange factor (GEF), functions to orient mitotic spindles during asymmetric cell divisions; however, whether Ric-8A has any role in mammalian cell division is unknown. We show here that Ric-8A and G alpha(i) function to orient the metaphase mitotic spindle of mammalian adherent cells. During mitosis, Ric-8A localized at the cell cortex, spindle poles, centromeres, central spindle, and midbody. Pertussis toxin proved to be a useful tool in these studies since it blocked the binding of Ric-8A to G alpha(i), thus preventing its GEF activity for G alpha(i). Linking Ric-8A signaling to mammalian cell division, treatment of cells with pertussis toxin, reduction of Ric-8A expression, or decreased G alpha(i) expression similarly affected metaphase cells. Each treatment impaired the localization of LGN (GSPM2), NuMA (microtubule binding nuclear mitotic apparatus protein), and dynein at the metaphase cell cortex and disturbed integrin-dependent mitotic spindle orientation. Live cell imaging of HeLa cells expressing green fluorescent protein-tubulin also revealed that reduced Ric-8A expression prolonged mitosis, caused occasional mitotic arrest, and decreased mitotic spindle movements. These data indicate that Ric-8A signaling leads to assembly of a cortical signaling complex that functions to orient the mitotic spindle.
Figures

Similar articles
-
Evidence for dynein and astral microtubule-mediated cortical release and transport of Gαi/LGN/NuMA complex in mitotic cells.Mol Biol Cell. 2013 Apr;24(7):901-13. doi: 10.1091/mbc.E12-06-0458. Epub 2013 Feb 6. Mol Biol Cell. 2013. PMID: 23389635 Free PMC article.
-
NuMA interacts with phosphoinositides and links the mitotic spindle with the plasma membrane.EMBO J. 2014 Aug 18;33(16):1815-30. doi: 10.15252/embj.201488147. Epub 2014 Jul 4. EMBO J. 2014. PMID: 24996901 Free PMC article.
-
NuMA phosphorylation by CDK1 couples mitotic progression with cortical dynein function.EMBO J. 2013 Sep 11;32(18):2517-29. doi: 10.1038/emboj.2013.172. Epub 2013 Aug 6. EMBO J. 2013. PMID: 23921553 Free PMC article.
-
Role of NuMA in vertebrate cells: review of an intriguing multifunctional protein.Front Biosci. 2006 Jan 1;11:1137-46. doi: 10.2741/1868. Front Biosci. 2006. PMID: 16146802 Review.
-
On the inscrutable role of Inscuteable: structural basis and functional implications for the competitive binding of NuMA and Inscuteable to LGN.Open Biol. 2012 Aug;2(8):120102. doi: 10.1098/rsob.120102. Open Biol. 2012. PMID: 22977735 Free PMC article. Review.
Cited by
-
MISP is a novel Plk1 substrate required for proper spindle orientation and mitotic progression.J Cell Biol. 2013 Mar 18;200(6):773-87. doi: 10.1083/jcb.201207050. J Cell Biol. 2013. PMID: 23509069 Free PMC article.
-
The nucleotide exchange factor Ric-8A is a chaperone for the conformationally dynamic nucleotide-free state of Gαi1.PLoS One. 2011;6(8):e23197. doi: 10.1371/journal.pone.0023197. Epub 2011 Aug 11. PLoS One. 2011. PMID: 21853086 Free PMC article.
-
The G protein α chaperone Ric-8 as a potential therapeutic target.Mol Pharmacol. 2015 Jan;87(1):52-63. doi: 10.1124/mol.114.094664. Epub 2014 Oct 15. Mol Pharmacol. 2015. PMID: 25319541 Free PMC article. Review.
-
The G protein alpha chaperone and guanine-nucleotide exchange factor RIC-8 regulates cilia morphogenesis in Caenorhabditis elegans sensory neurons.PLoS Genet. 2023 Nov 1;19(11):e1011015. doi: 10.1371/journal.pgen.1011015. eCollection 2023 Nov. PLoS Genet. 2023. PMID: 37910589 Free PMC article.
-
MAP4 and CLASP1 operate as a safety mechanism to maintain a stable spindle position in mitosis.Nat Cell Biol. 2011 Aug 7;13(9):1040-50. doi: 10.1038/ncb2297. Nat Cell Biol. 2011. PMID: 21822276
References
-
- Afshar, K., F. S. Willard, K. Colombo, C. A. Johnston, C. R. McCudden, D. P. Siderovski, and P. Gonczy. 2004. RIC-8 is required for GPR-1/2-dependent Gα function during asymmetric division of Caenorhabditis elegans embryos. Cell 119:219-230. - PubMed
-
- Afshar, K., F. S. Willard, K. Colombo, D. P. Siderovski, and P. Gonczy. 2005. Cortical localization of the Gα protein GPA-16 requires RIC-8 function during Caenorhabditis elegans asymmetric cell division. Development 132:4449-4459. - PubMed
-
- Blumer, J. B., R. Kuriyama, T. W. Gettys, and S. M. Lanier. 2006. The G-protein regulatory (GPR) motif-containing Leu-Gly-Asn-enriched protein (LGN) and Giα3 influence cortical positioning of the mitotic spindle poles at metaphase in symmetrically dividing mammalian cells. Eur. J. Cell Biol. 85:1233-1240. - PubMed
-
- Busson, S., D. Dujardin, A. Moreau, J. Dompierre, and J. R. De Mey. 1998. Dynein and dynactin are localized to astral microtubules and at cortical sites in mitotic epithelial cells. Curr. Biol. 8:541-544. - PubMed
-
- Couwenbergs, C., A. C. Spilker, and M. Gotta. 2004. Control of embryonic spindle positioning and Gα activity by Caenorhabditis elegans RIC-8. Curr. Biol. 14:1871-1876. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
