Reversible association of ribulose-1, 5-bisphosphate carboxylase/oxygenase activase with the thylakoid membrane depends upon the ATP level and pH in rice without heat stress
- PMID: 20478969
- PMCID: PMC2892142
- DOI: 10.1093/jxb/erq122
Reversible association of ribulose-1, 5-bisphosphate carboxylase/oxygenase activase with the thylakoid membrane depends upon the ATP level and pH in rice without heat stress
Abstract
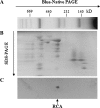
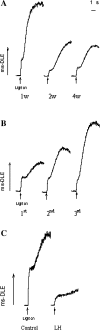
Ribulose-1, 5-bisphosphate carboxylase/oxygenase (Rubisco) activase (RCA) in the thylakoid membrane (TM) has been shown to play a role in protection and regulation of photosynthesis under moderate heat stress. However, the physiological significance of RCA bound to the TM (TM-RCA) without heat stress remains unknown. In this study, it is first shown, using experiments in vivo, that the TM-RCA varies in rice leaves at different development stages, under different environmental conditions, and in a rice mutant. Furthermore, it is shown that the amount of TM-RCA always increased when the Rubisco activation state and the pH gradient across the TM (DeltapH) decreased. It was then demonstrated in vitro that the RCA bound dynamically to TM and the amount of TM-RCA increased during Rubisco activation. A high level of ATP and a high pH value promoted the dissociation of RCA from the TM. Both the RCA association with and dissociation from the TM showed conformational changes related to the ATP level or pH as indicated by the changes in fluorescence intensity of 1-anilinonaphthalene-8-sulphonic acid (ANS) binding to RCA. These results suggest that the reversible association of RCA with the TM is ATP and pH (or DeltapH) dependent; it might be involved in the RCA activation of Rubisco, in addition to the previously discovered role in the protection and regulation of photosynthesis under heat stress.
Figures
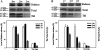
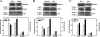



Similar articles
-
Heat tolerance in a wild Oryza species is attributed to maintenance of Rubisco activation by a thermally stable Rubisco activase ortholog.New Phytol. 2016 Aug;211(3):899-911. doi: 10.1111/nph.13963. Epub 2016 May 5. New Phytol. 2016. PMID: 27145723
-
Antisense inhibition of Rubisco activase increases Rubisco content and alters the proportion of Rubisco activase in stroma and thylakoids in chloroplasts of rice leaves.Ann Bot. 2006 May;97(5):739-44. doi: 10.1093/aob/mcl025. Epub 2006 Feb 14. Ann Bot. 2006. PMID: 16478766 Free PMC article.
-
Rubisco Activase Is Also a Multiple Responder to Abiotic Stresses in Rice.PLoS One. 2015 Oct 19;10(10):e0140934. doi: 10.1371/journal.pone.0140934. eCollection 2015. PLoS One. 2015. PMID: 26479064 Free PMC article.
-
Recent developments in the engineering of Rubisco activase for enhanced crop yield.Biochem Soc Trans. 2023 Apr 26;51(2):627-637. doi: 10.1042/BST20221281. Biochem Soc Trans. 2023. PMID: 36929563 Review.
-
Improving plant heat tolerance through modification of Rubisco activase in C3 plants to secure crop yield and food security in a future warming world.J Exp Bot. 2023 Jan 11;74(2):591-599. doi: 10.1093/jxb/erac340. J Exp Bot. 2023. PMID: 35981868 Review.
Cited by
-
Plant tolerance to high temperature in a changing environment: scientific fundamentals and production of heat stress-tolerant crops.Front Plant Sci. 2013 Jul 31;4:273. doi: 10.3389/fpls.2013.00273. eCollection 2013. Front Plant Sci. 2013. PMID: 23914193 Free PMC article.
-
Elevated CO2 concentration promotes photosynthesis of grape (Vitis vinifera L. cv. 'Pinot noir') plantlet in vitro by regulating RbcS and Rca revealed by proteomic and transcriptomic profiles.BMC Plant Biol. 2019 Jan 29;19(1):42. doi: 10.1186/s12870-019-1644-y. BMC Plant Biol. 2019. PMID: 30696402 Free PMC article.
-
Exogenous Putrescine Increases Heat Tolerance in Tomato Seedlings by Regulating Chlorophyll Metabolism and Enhancing Antioxidant Defense Efficiency.Plants (Basel). 2022 Apr 11;11(8):1038. doi: 10.3390/plants11081038. Plants (Basel). 2022. PMID: 35448766 Free PMC article.
-
Proteome and Acetyl-Proteome Profiling of Camellia sinensis cv. 'Anjin Baicha' during Periodic Albinism Reveals Alterations in Photosynthetic and Secondary Metabolite Biosynthetic Pathways.Front Plant Sci. 2017 Dec 11;8:2104. doi: 10.3389/fpls.2017.02104. eCollection 2017. Front Plant Sci. 2017. PMID: 29312376 Free PMC article.
-
RICE MORPHOLOGY DETERMINANT encodes the type II formin FH5 and regulates rice morphogenesis.Plant Cell. 2011 Feb;23(2):681-700. doi: 10.1105/tpc.110.081349. Epub 2011 Feb 9. Plant Cell. 2011. PMID: 21307283 Free PMC article.
References
-
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry. 1976;72:248–254. - PubMed

