Does accumulation of advanced glycation end products contribute to the aging phenotype?
- PMID: 20478906
- PMCID: PMC2920582
- DOI: 10.1093/gerona/glq074
Does accumulation of advanced glycation end products contribute to the aging phenotype?
Abstract
Background: Aging is a complex multifactorial process characterized by accumulation of deleterious changes in cells and tissues, progressive deterioration of structural integrity and physiological function across multiple organ systems, and increased risk of death.
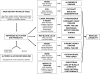
Methods: We conducted a review of the scientific literature on the relationship of advanced glycation end products (AGEs) with aging. AGEs are a heterogeneous group of bioactive molecules that are formed by the nonenzymatic glycation of proteins, lipids, and nucleic acids.
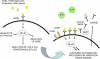
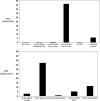
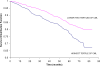
Results: Humans are exposed to AGEs produced in the body, especially in individuals with abnormal glucose metabolism, and AGEs ingested in foods. AGEs cause widespread damage to tissues through upregulation of inflammation and cross-linking of collagen and other proteins. AGEs have been shown to adversely affect virtually all cells, tissues, and organ systems. Recent epidemiological studies demonstrate that elevated circulating AGEs are associated with increased risk of developing many chronic diseases that disproportionally affect older individuals.
Conclusions: Based on these data, we propose that accumulation of AGEs accelerate the multisystem functional decline that occurs with aging, and therefore contribute to the aging phenotype. Exposure to AGEs can be reduced by restriction of dietary intake of AGEs and drug treatment with AGE inhibitors and AGE breakers. Modification of intake and circulating levels of AGEs may be a possible strategy to promote health in old age, especially because most Western foods are processed at high temperature and are rich in AGEs.
Figures

Similar articles
-
Identifying advanced glycation end products as a major source of oxidants in aging: implications for the management and/or prevention of reduced renal function in elderly persons.Semin Nephrol. 2009 Nov;29(6):594-603. doi: 10.1016/j.semnephrol.2009.07.013. Semin Nephrol. 2009. PMID: 20006791 Free PMC article.
-
Aging and glycoxidant stress.Hormones (Athens). 2008 Apr-Jun;7(2):123-32. doi: 10.1007/BF03401503. Hormones (Athens). 2008. PMID: 18477549 Review.
-
Advanced glycation end products and insulin resistance.Curr Pharm Des. 2008;14(10):987-9. doi: 10.2174/138161208784139747. Curr Pharm Des. 2008. PMID: 18473850 Review.
-
Advanced glycation end products (AGEs) and their involvement in liver disease.Curr Pharm Des. 2008;14(10):969-72. doi: 10.2174/138161208784139701. Curr Pharm Des. 2008. PMID: 18473847 Review.
-
Oral glycotoxins determine the effects of calorie restriction on oxidant stress, age-related diseases, and lifespan.Am J Pathol. 2008 Aug;173(2):327-36. doi: 10.2353/ajpath.2008.080152. Epub 2008 Jul 3. Am J Pathol. 2008. PMID: 18599606 Free PMC article.
Cited by
-
Effect of tangweian jianji on upper gastrointestinal remodeling in streptozotocin-induced diabetic rats.World J Gastroenterol. 2012 Sep 21;18(35):4875-84. doi: 10.3748/wjg.v18.i35.4875. World J Gastroenterol. 2012. PMID: 23002359 Free PMC article.
-
Research Progress on Frailty in Elderly People.Clin Interv Aging. 2024 Aug 29;19:1493-1505. doi: 10.2147/CIA.S474547. eCollection 2024. Clin Interv Aging. 2024. PMID: 39224708 Free PMC article. Review.
-
Honghua Xiaoyao tablet combined with estradiol improves ovarian function in D-galactose-induced aging mice by reducing apoptosis and affecting the release of reproductive hormones: an in vivo study.Front Pharmacol. 2024 Jun 6;15:1394941. doi: 10.3389/fphar.2024.1394941. eCollection 2024. Front Pharmacol. 2024. PMID: 38903998 Free PMC article.
-
Synbiotic Bacillus megaterium DSM 32963 and n-3 PUFA Salt Composition Elevates Pro-Resolving Lipid Mediator Levels in Healthy Subjects: A Randomized Controlled Study.Nutrients. 2024 Apr 30;16(9):1354. doi: 10.3390/nu16091354. Nutrients. 2024. PMID: 38732601 Free PMC article. Clinical Trial.
-
On the Relationship between Energy Metabolism, Proteostasis, Aging and Parkinson's Disease: Possible Causative Role of Methylglyoxal and Alleviative Potential of Carnosine.Aging Dis. 2017 May 2;8(3):334-345. doi: 10.14336/AD.2016.1030. eCollection 2017 May. Aging Dis. 2017. PMID: 28580188 Free PMC article. Review.
References
-
- Cho SJ, Roman G, Yeboah F, Konishi Y. The road to advanced glycation end products: a mechanistic perspective. Curr Med Chem. 2007;14:1653–1671. - PubMed
-
- Maillard LC. Action des acides aminés sur les sucres: formation des mélanoïdines par voie méthodique. C R Acad Sci. 1912;154:66–68.
-
- Trivelli LA, Tanney HM, Lai HT. Hemoglobin components in patients with diabetes mellitus. N Engl J Med. 1971;284:353–357. - PubMed
-
- Ulrich P, Cerami A. Protein glycation, diabetes, and aging. Recent Prog Horm Res. 2001;56:1–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

