The human collagen beta(1-O)galactosyltransferase, GLT25D1, is a soluble endoplasmic reticulum localized protein
- PMID: 20470363
- PMCID: PMC2877668
- DOI: 10.1186/1471-2121-11-33
The human collagen beta(1-O)galactosyltransferase, GLT25D1, is a soluble endoplasmic reticulum localized protein
Abstract
Background: Glycosyl transferases transfer glycosyl groups onto their substrate. Localization partially defines their function. Glycosyl transferase 25 domain 1 (GLT25D1) was recently shown to have galactosyltransferase activity towards collagens and another well known substrate, mannose binding lectin (MBL). To gain more insight in the role of galactosylation of lysines in the Gly-X-Lys repeats of collagenous proteins, we investigated the subcellular localization of GLT25D1.
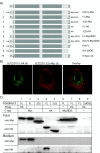
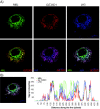
Results: Immunofluorescence analysis of GLT25D1 expressed in the human hepatoma cell line (Huh7), revealed a perinuclear lattice like staining, resembling localization to the endoplasmic reticulum (ER). Possible targeting signals, an N-terminal signal sequence and a C-terminal ER-retention signal, were identified using prediction programs. These signals were then investigated by constructing a series of epitope-tagged forms of GLT25D1 that were analyzed by immunofluorescence and western blotting. In agreement with the predictions our results show that GLT25D1 is directed to the ER lumen as a soluble protein and retained there. Moreover, using two endoglycosidase enzymes EndoH and EndoF, we demonstrate that the putative bi-functional glycosyl transferase itself is a glycoprotein. Additionally we examined co-localization of GLT25D1 with MBL and lysyl hydroxylase 3 (LH3, PLOD3), which is a protein able to catalyze hydroxylation of lysine residues before they can be glycosylated. We demonstrate overlapping localization patterns of GLT25D1, MBL and LH3.
Conclusions: Taken together our data indicate that galactosylation of collagenous proteins by the soluble GLT25D1 occurs in the early secretory pathway.
Figures



Similar articles
-
Core glycosylation of collagen is initiated by two beta(1-O)galactosyltransferases.Mol Cell Biol. 2009 Feb;29(4):943-52. doi: 10.1128/MCB.02085-07. Epub 2008 Dec 15. Mol Cell Biol. 2009. PMID: 19075007 Free PMC article.
-
Involvement of LH3 and GLT25D1 for glucosyl-galactosyl-hydroxylation on non-collagen-like domain of FGL1.Biochem Biophys Res Commun. 2021 Jun 30;560:93-98. doi: 10.1016/j.bbrc.2021.04.128. Epub 2021 May 10. Biochem Biophys Res Commun. 2021. PMID: 33984770
-
Lysyl hydroxylase 3 modifies lysine residues to facilitate oligomerization of mannan-binding lectin.PLoS One. 2014 Nov 24;9(11):e113498. doi: 10.1371/journal.pone.0113498. eCollection 2014. PLoS One. 2014. PMID: 25419660 Free PMC article.
-
Expanding the lysyl hydroxylase toolbox: new insights into the localization and activities of lysyl hydroxylase 3 (LH3).J Cell Physiol. 2007 Aug;212(2):323-9. doi: 10.1002/jcp.21036. J Cell Physiol. 2007. PMID: 17516569 Review.
-
Identification of Regulatory Molecular "Hot Spots" for LH/PLOD Collagen Glycosyltransferase Activity.Int J Mol Sci. 2023 Jul 7;24(13):11213. doi: 10.3390/ijms241311213. Int J Mol Sci. 2023. PMID: 37446392 Free PMC article. Review.
Cited by
-
Molecular Characterization of Collagen Hydroxylysine O-Glycosylation by Mass Spectrometry: Current Status.Aust J Chem. 2013 Jul 18;66(7):760-769. doi: 10.1071/CH13174. Aust J Chem. 2013. PMID: 25414518 Free PMC article.
-
Roles of PLODs in Collagen Synthesis and Cancer Progression.Front Cell Dev Biol. 2018 Jun 28;6:66. doi: 10.3389/fcell.2018.00066. eCollection 2018. Front Cell Dev Biol. 2018. PMID: 30003082 Free PMC article. Review.
-
Identification of domains and amino acids essential to the collagen galactosyltransferase activity of GLT25D1.PLoS One. 2011;6(12):e29390. doi: 10.1371/journal.pone.0029390. Epub 2011 Dec 21. PLoS One. 2011. PMID: 22216269 Free PMC article.
-
Collagen beta (1-O) galactosyltransferase 1 (GLT25D1) is required for the secretion of high molecular weight adiponectin and affects lipid accumulation.Biosci Rep. 2017 May 17;37(3):BSR20170105. doi: 10.1042/BSR20170105. Print 2017 Jun 30. Biosci Rep. 2017. PMID: 28428430 Free PMC article.
-
Collagen Accumulation in Osteosarcoma Cells lacking GLT25D1 Collagen Galactosyltransferase.J Biol Chem. 2016 Aug 26;291(35):18514-24. doi: 10.1074/jbc.M116.723379. Epub 2016 Jul 11. J Biol Chem. 2016. PMID: 27402836 Free PMC article.
References
-
- Brodsky B, Persikov AV. Molecular structure of the collagen triple helix. Adv Protein Chem. 2005;70:301–339. full_text. - PubMed
-
- Colley KJ, Baenziger JU. Identification of the post-translational modifications of the core-specific lectin. The core-specific lectin contains hydroxyproline, hydroxylysine, and glucosylgalactosylhydroxylysine residues. J Biol Chem. 1987;262:10290–10295. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

