Recognition of the ring-opened state of proliferating cell nuclear antigen by replication factor C promotes eukaryotic clamp-loading
- PMID: 20455582
- PMCID: PMC2876781
- DOI: 10.1021/ja100365x
Recognition of the ring-opened state of proliferating cell nuclear antigen by replication factor C promotes eukaryotic clamp-loading
Abstract
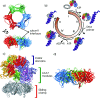
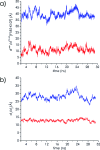
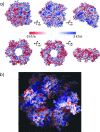
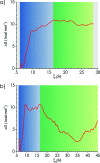
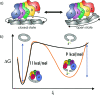
Proliferating cell nuclear antigen (PCNA, sliding clamp) is a toroidal-shaped protein that encircles DNA and plays a pivotal role in DNA replication, modification and repair. To perform its vital functions, the clamp has to be opened and resealed at primer-template junctions by a clamp loader molecular machine, replication factor C (RFC). The mechanism of this process constitutes a significant piece in the puzzle of processive DNA replication. We show that upon clamp opening the RFC/PCNA complex undergoes a large conformational rearrangement, leading to the formation of an extended interface between the clamp and RFC. Binding of ring-open PCNA to all five RFC subunits transforms the free-energy landscape underlying the closed- to open state transition, trapping PCNA in an open conformation. Careful comparison of free-energy profiles for clamp opening in the presence and absence of RFC allowed us to substantiate the role of RFC in the initial stage of the clamp-loading cycle. RFC does not appreciably destabilize the closed state of PCNA. Instead, the function of the clamp loader is dependent on the selective stabilization of the open conformation of the clamp.
Figures

Similar articles
-
A central swivel point in the RFC clamp loader controls PCNA opening and loading on DNA.J Mol Biol. 2012 Feb 17;416(2):163-75. doi: 10.1016/j.jmb.2011.12.017. Epub 2011 Dec 13. J Mol Biol. 2012. PMID: 22197374 Free PMC article.
-
Cryo-EM structures reveal high-resolution mechanism of a DNA polymerase sliding clamp loader.Elife. 2022 Feb 18;11:e74175. doi: 10.7554/eLife.74175. Elife. 2022. PMID: 35179493 Free PMC article.
-
Kinetic analysis of PCNA clamp binding and release in the clamp loading reaction catalyzed by Saccharomyces cerevisiae replication factor C.Biochim Biophys Acta. 2015 Jan;1854(1):31-8. doi: 10.1016/j.bbapap.2014.09.019. Epub 2014 Oct 23. Biochim Biophys Acta. 2015. PMID: 25450506 Free PMC article.
-
Functions of Multiple Clamp and Clamp-Loader Complexes in Eukaryotic DNA Replication.Adv Exp Med Biol. 2017;1042:135-162. doi: 10.1007/978-981-10-6955-0_7. Adv Exp Med Biol. 2017. PMID: 29357057 Review.
-
The PCNA-RFC families of DNA clamps and clamp loaders.Prog Nucleic Acid Res Mol Biol. 2004;78:227-60. doi: 10.1016/S0079-6603(04)78006-X. Prog Nucleic Acid Res Mol Biol. 2004. PMID: 15210332 Review.
Cited by
-
Replication factor C is a more effective proliferating cell nuclear antigen (PCNA) opener than the checkpoint clamp loader, Rad24-RFC.J Biol Chem. 2012 Jan 13;287(3):2203-9. doi: 10.1074/jbc.C111.318899. Epub 2011 Nov 24. J Biol Chem. 2012. PMID: 22115746 Free PMC article.
-
Clamp loader ATPases and the evolution of DNA replication machinery.BMC Biol. 2012 Apr 20;10:34. doi: 10.1186/1741-7007-10-34. BMC Biol. 2012. PMID: 22520345 Free PMC article.
-
A central swivel point in the RFC clamp loader controls PCNA opening and loading on DNA.J Mol Biol. 2012 Feb 17;416(2):163-75. doi: 10.1016/j.jmb.2011.12.017. Epub 2011 Dec 13. J Mol Biol. 2012. PMID: 22197374 Free PMC article.
-
XPB and XPD helicases in TFIIH orchestrate DNA duplex opening and damage verification to coordinate repair with transcription and cell cycle via CAK kinase.DNA Repair (Amst). 2011 Jul 15;10(7):697-713. doi: 10.1016/j.dnarep.2011.04.028. Epub 2011 May 14. DNA Repair (Amst). 2011. PMID: 21571596 Free PMC article. Review.
-
P-loop conformation governed crizotinib resistance in G2032R-mutated ROS1 tyrosine kinase: clues from free energy landscape.PLoS Comput Biol. 2014 Jul 17;10(7):e1003729. doi: 10.1371/journal.pcbi.1003729. eCollection 2014 Jul. PLoS Comput Biol. 2014. PMID: 25033171 Free PMC article.
References
-
- Benkovic S. J.; Valentine A. M.; Salinas F. Annu. Rev. Biochem. 2001, 70, 181–208. - PubMed
-
- O’Donnell M. J. Biol. Chem. 2006, 281, 10653–10656. - PubMed
-
- Johnson A.; O’Donnell M. Annu. Rev. Biochem. 2005, 74, 283–315. - PubMed
-
- Burgers P. M. J. Chromosoma 1998, 107, 218–227. - PubMed
-
- Kelman Z. Oncogene 1997, 14, 629–640. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

