Nucleosome interaction surface of linker histone H1c is distinct from that of H1(0)
- PMID: 20444700
- PMCID: PMC2898364
- DOI: 10.1074/jbc.M110.108639
Nucleosome interaction surface of linker histone H1c is distinct from that of H1(0)
Abstract
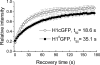
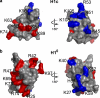
The fully organized structure of the eukaryotic nucleosome remains unsolved, in part due to limited information regarding the binding site of the H1 or linker histone. The central globular domain of H1 is believed to interact with the nucleosome core at or near the dyad and to bind at least two strands of DNA. We utilized site-directed mutagenesis and in vivo photobleaching to identify residues that contribute to the binding of the globular domain of the somatic H1 subtype H1c to the nucleosome. As was previously observed for the H1(0) subtype, the binding residues for H1c are clustered on the surface of one face of the domain. Despite considerable structural conservation between the globular domains of these two subtypes, the locations of the binding sites identified for H1c are distinct from those of H1(0). We suggest that the globular domains of these two linker histone subtypes will bind to the nucleosome with distinct orientations that may contribute to higher order chromatin structure heterogeneity or to differences in dynamic interactions with other DNA or chromatin-binding proteins.
Figures

Similar articles
-
N- and C-terminal domains determine differential nucleosomal binding geometry and affinity of linker histone isotypes H1(0) and H1c.J Biol Chem. 2012 Apr 6;287(15):11778-87. doi: 10.1074/jbc.M111.312819. Epub 2012 Feb 10. J Biol Chem. 2012. PMID: 22334665 Free PMC article.
-
Mapping the interaction surface of linker histone H1(0) with the nucleosome of native chromatin in vivo.Nat Struct Mol Biol. 2006 Mar;13(3):250-5. doi: 10.1038/nsmb1050. Epub 2006 Feb 5. Nat Struct Mol Biol. 2006. PMID: 16462749 Free PMC article.
-
A Small Number of Residues Can Determine if Linker Histones Are Bound On or Off Dyad in the Chromatosome.J Mol Biol. 2016 Oct 9;428(20):3948-3959. doi: 10.1016/j.jmb.2016.08.016. Epub 2016 Aug 21. J Mol Biol. 2016. PMID: 27558112 Free PMC article.
-
Chromatin structures condensed by linker histones.Essays Biochem. 2019 Apr 23;63(1):75-87. doi: 10.1042/EBC20180056. Print 2019 Apr 23. Essays Biochem. 2019. PMID: 31015384 Review.
-
The H1 linker histones: multifunctional proteins beyond the nucleosomal core particle.EMBO Rep. 2015 Nov;16(11):1439-53. doi: 10.15252/embr.201540749. Epub 2015 Oct 15. EMBO Rep. 2015. PMID: 26474902 Free PMC article. Review.
Cited by
-
A brief review of nucleosome structure.FEBS Lett. 2015 Oct 7;589(20 Pt A):2914-22. doi: 10.1016/j.febslet.2015.05.016. Epub 2015 May 14. FEBS Lett. 2015. PMID: 25980611 Free PMC article. Review.
-
What is the role of histone H1 heterogeneity? A functional model emerges from a 50 year mystery.AIMS Biophys. 2015;2(4):724-772. doi: 10.3934/biophy.2015.4.724. Epub 2015 Nov 16. AIMS Biophys. 2015. PMID: 31289748 Free PMC article.
-
Nuclear and nucleolar activity of linker histone variant H1.0.Cell Mol Biol Lett. 2016 Aug 24;21:15. doi: 10.1186/s11658-016-0014-0. eCollection 2016. Cell Mol Biol Lett. 2016. PMID: 28536618 Free PMC article. Review.
-
Conformational selection and dynamic adaptation upon linker histone binding to the nucleosome.Nucleic Acids Res. 2016 Aug 19;44(14):6599-613. doi: 10.1093/nar/gkw514. Epub 2016 Jun 7. Nucleic Acids Res. 2016. PMID: 27270081 Free PMC article.
-
Elucidating the influence of linker histone variants on chromatosome dynamics and energetics.Nucleic Acids Res. 2020 Apr 17;48(7):3591-3604. doi: 10.1093/nar/gkaa121. Nucleic Acids Res. 2020. PMID: 32128577 Free PMC article.
References
-
- Wolffe A. (1998) Chromatin: Structure and Function, 3rd Ed., Academic Press, San Diego
-
- van Holde K. (1989) Chromatin, Springer-Verlag, New York
-
- Luger K., Mäder A. W., Richmond R. K., Sargent D. F., Richmond T. J. (1997) Nature 389, 251–260 - PubMed
-
- Woodcock C. L. (2006) Curr. Opin. Struct. Biol. 16, 213–220 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

