G domain dimerization controls dynamin's assembly-stimulated GTPase activity
- PMID: 20428113
- PMCID: PMC2879890
- DOI: 10.1038/nature09032
G domain dimerization controls dynamin's assembly-stimulated GTPase activity
Abstract
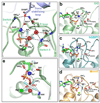
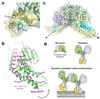
Dynamin is an atypical GTPase that catalyses membrane fission during clathrin-mediated endocytosis. The mechanisms of dynamin's basal and assembly-stimulated GTP hydrolysis are unknown, though both are indirectly influenced by the GTPase effector domain (GED). Here we present the 2.0 A resolution crystal structure of a human dynamin 1-derived minimal GTPase-GED fusion protein, which was dimeric in the presence of the transition state mimic GDP.AlF(4)(-).The structure reveals dynamin's catalytic machinery and explains how assembly-stimulated GTP hydrolysis is achieved through G domain dimerization. A sodium ion present in the active site suggests that dynamin uses a cation to compensate for the developing negative charge in the transition state in the absence of an arginine finger. Structural comparison to the rat dynamin G domain reveals key conformational changes that promote G domain dimerization and stimulated hydrolysis. The structure of the GTPase-GED fusion protein dimer provides insight into the mechanisms underlying dynamin-catalysed membrane fission.
Figures



Similar articles
-
An intramolecular signaling element that modulates dynamin function in vitro and in vivo.Mol Biol Cell. 2009 Aug;20(15):3561-71. doi: 10.1091/mbc.e09-04-0318. Epub 2009 Jun 10. Mol Biol Cell. 2009. PMID: 19515832 Free PMC article.
-
Domain structure and intramolecular regulation of dynamin GTPase.EMBO J. 1997 Nov 17;16(22):6676-83. doi: 10.1093/emboj/16.22.6676. EMBO J. 1997. PMID: 9362482 Free PMC article.
-
The dynamin middle domain is critical for tetramerization and higher-order self-assembly.EMBO J. 2007 Jan 24;26(2):559-66. doi: 10.1038/sj.emboj.7601491. Epub 2006 Dec 14. EMBO J. 2007. PMID: 17170701 Free PMC article.
-
Dynamin and its role in membrane fission.Annu Rev Cell Dev Biol. 2000;16:483-519. doi: 10.1146/annurev.cellbio.16.1.483. Annu Rev Cell Dev Biol. 2000. PMID: 11031245 Free PMC article. Review.
-
It takes two to tango: regulation of G proteins by dimerization.Nat Rev Mol Cell Biol. 2009 Jun;10(6):423-9. doi: 10.1038/nrm2689. Epub 2009 May 8. Nat Rev Mol Cell Biol. 2009. PMID: 19424291 Review.
Cited by
-
Molecular mechanisms of mitochondrial dynamics.Nat Rev Mol Cell Biol. 2024 Oct 17. doi: 10.1038/s41580-024-00785-1. Online ahead of print. Nat Rev Mol Cell Biol. 2024. PMID: 39420231 Review.
-
Rab3B Proteins: Cellular Functions, Regulatory Mechanisms, and Potential as a Cancer Therapy Target.Cell Biochem Biophys. 2024 Sep 25. doi: 10.1007/s12013-024-01549-6. Online ahead of print. Cell Biochem Biophys. 2024. PMID: 39320613 Review.
-
Identification of SLC25A46 interaction interfaces with mitochondrial membrane fusogens Opa1 and Mfn2.J Biol Chem. 2024 Aug 31;300(10):107740. doi: 10.1016/j.jbc.2024.107740. Online ahead of print. J Biol Chem. 2024. PMID: 39222684 Free PMC article.
-
Fast and deep phosphoproteome analysis with the Orbitrap Astral mass spectrometer.Nat Commun. 2024 Aug 15;15(1):7016. doi: 10.1038/s41467-024-51274-0. Nat Commun. 2024. PMID: 39147754 Free PMC article.
-
Identification of drug-like molecules targeting the ATPase activity of dynamin-like EHD4.PLoS One. 2024 Jul 29;19(7):e0302704. doi: 10.1371/journal.pone.0302704. eCollection 2024. PLoS One. 2024. PMID: 39074100 Free PMC article.
References
-
- Praefcke GJ, McMahon HT. The dynamin superfamily: universal membrane tubulation and fission molecules? Nat. Rev. Mol. Cell Biol. 2004;5:133–147. - PubMed
-
- Stowell MH, Marks B, Wigge P, McMahon HT. Nucleotide-dependent conformational changes in dynamin: evidence for a mechanochemical molecular spring. Nat. Cell Biol. 1999;1:27–32. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

