Priming of hippocampal population bursts by individual perisomatic-targeting interneurons
- PMID: 20427657
- PMCID: PMC3763476
- DOI: 10.1523/JNEUROSCI.3962-09.2010
Priming of hippocampal population bursts by individual perisomatic-targeting interneurons
Abstract
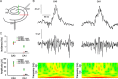
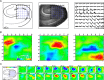
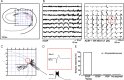
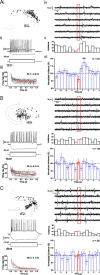
Hippocampal population bursts ("sharp wave-ripples") occur during rest and slow-wave sleep and are thought to be important for memory consolidation. The cellular mechanisms involved are incompletely understood. Here we investigated the cellular mechanisms underlying the initiation of sharp waves using a hippocampal slice model. To this end, we used a combination of field recordings with planar multielectrode arrays and whole-cell patch-clamp recordings of individual anatomically identified pyramidal neurons and interneurons. We found that GABA(A) receptor-mediated inhibition is necessary for sharp wave generation. Moreover, the activity of individual perisomatic-targeting interneurons can both suppress, and subsequently enhance, the local generation of sharp waves. Finally, we show that this is achieved by the tight control of local excitation and inhibition by perisomatic-targeting interneurons. These results suggest that perisomatic-targeting interneurons assist in selecting the subset of pyramidal neurons that initiate each hippocampal sharp wave-ripple.
Figures



Similar articles
-
The hippocampal CA3 region can generate two distinct types of sharp wave-ripple complexes, in vitro.Hippocampus. 2015 Feb;25(2):169-86. doi: 10.1002/hipo.22361. Epub 2014 Sep 25. Hippocampus. 2015. PMID: 25209976
-
Synaptic GABA(A) activation inhibits AMPA-kainate receptor-mediated bursting in the newborn (P0-P2) rat hippocampus.J Neurophysiol. 2000 Jan;83(1):359-66. doi: 10.1152/jn.2000.83.1.359. J Neurophysiol. 2000. PMID: 10634879
-
Properties and dynamics of inhibitory synaptic communication within the CA3 microcircuits of pyramidal cells and interneurons expressing parvalbumin or cholecystokinin.J Physiol. 2016 Jul 1;594(13):3745-74. doi: 10.1113/JP272231. Epub 2016 May 5. J Physiol. 2016. PMID: 27038232 Free PMC article.
-
[Neural mechanism underlying generation of synchronous oscillations in hippocampal network].Brain Nerve. 2008 Jul;60(7):755-62. Brain Nerve. 2008. PMID: 18646615 Review. Japanese.
-
[Hippocampal ripple oscillations (200 Hz) in mechanisms of memory consolidation].Usp Fiziol Nauk. 2002 Oct-Dec;33(4):34-42. Usp Fiziol Nauk. 2002. PMID: 12449805 Review. Russian.
Cited by
-
Selective reduction of AMPA currents onto hippocampal interneurons impairs network oscillatory activity.PLoS One. 2012;7(6):e37318. doi: 10.1371/journal.pone.0037318. Epub 2012 Jun 4. PLoS One. 2012. PMID: 22675480 Free PMC article.
-
Assessment of Methods for the Intracellular Blockade of GABAA Receptors.PLoS One. 2016 Aug 8;11(8):e0160900. doi: 10.1371/journal.pone.0160900. eCollection 2016. PLoS One. 2016. PMID: 27501143 Free PMC article.
-
Neural syntax: cell assemblies, synapsembles, and readers.Neuron. 2010 Nov 4;68(3):362-85. doi: 10.1016/j.neuron.2010.09.023. Neuron. 2010. PMID: 21040841 Free PMC article. Review.
-
Extracellular calcium controls the expression of two different forms of ripple-like hippocampal oscillations.J Neurosci. 2014 Feb 19;34(8):2989-3004. doi: 10.1523/JNEUROSCI.2826-13.2014. J Neurosci. 2014. PMID: 24553939 Free PMC article.
-
Hippocampal GABAergic interneurons and memory.Neuron. 2023 Oct 18;111(20):3154-3175. doi: 10.1016/j.neuron.2023.06.016. Epub 2023 Jul 18. Neuron. 2023. PMID: 37467748 Free PMC article. Review.
References
-
- Behrens CJ, van den Boom LP, de Hoz L, Friedman A, Heinemann U. Induction of sharp wave-ripple complexes in vitro and reorganization of hippocampal networks. Nat Neurosci. 2005;8:1560–1567. - PubMed
-
- Both M, Bähner F, von Bohlen und Halbach O, Draguhn A. Propagation of specific network patterns through the mouse hippocampus. Hippocampus. 2008;18:899–908. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
