Evolution of CASK into a Mg2+-sensitive kinase
- PMID: 20424264
- PMCID: PMC3286871
- DOI: 10.1126/scisignal.2000800
Evolution of CASK into a Mg2+-sensitive kinase
Abstract
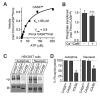
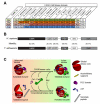
All known protein kinases, except CASK [calcium/calmodulin (CaM)-activated serine-threonine kinase], require magnesium ions (Mg(2+)) to stimulate the transfer of a phosphate from adenosine 5'-triphosphate (ATP) to a protein substrate. The CaMK (calcium/calmodulin-dependent kinase) domain of CASK shows activity in the absence of Mg(2+); indeed, it is inhibited by divalent ions including Mg(2+). Here, we converted the Mg(2+)-inhibited wild-type CASK kinase (CASK(WT)) into a Mg(2+)-stimulated kinase (CASK(4M)) by substituting four residues within the ATP-binding pocket. Crystal structures of CASK(4M) with and without bound nucleotide and Mn(2+), together with kinetic analyses, demonstrated that Mg(2+) accelerates catalysis of CASK(4M) by stabilizing the transition state, enhancing the leaving group properties of adenosine 5'-diphosphate, and indirectly shifting the position of the gamma-phosphate of ATP. Phylogenetic analysis revealed that the four residues conferring Mg(2+)-mediated stimulation were substituted from CASK during early animal evolution, converting a primordial, Mg(2+)-coordinating form of CASK into a Mg(2+)-inhibited kinase. This emergence of Mg(2+) sensitivity (inhibition by Mg(2+)) conferred regulation of CASK activity by divalent cations, in parallel with the evolution of the animal nervous systems.
Figures
Similar articles
-
CASK Functions as a Mg2+-independent neurexin kinase.Cell. 2008 Apr 18;133(2):328-39. doi: 10.1016/j.cell.2008.02.036. Cell. 2008. PMID: 18423203 Free PMC article.
-
The substitution of calcium for magnesium in H+,K+-ATPase catalytic cycle. Evidence for two actions of divalent cations.J Biol Chem. 1989 Nov 5;264(31):18512-9. J Biol Chem. 1989. PMID: 2553712
-
Divalent metal ions control activity and inhibition of protein kinases.Metallomics. 2017 Nov 15;9(11):1576-1584. doi: 10.1039/c7mt00204a. Metallomics. 2017. PMID: 29043344
-
Structural constraints and functional divergences in CASK evolution.Biochem Soc Trans. 2013 Aug;41(4):1017-22. doi: 10.1042/BST20130061. Biochem Soc Trans. 2013. PMID: 23863172 Review.
-
Catalytic mechanisms and regulation of protein kinases.Methods Enzymol. 2014;548:1-21. doi: 10.1016/B978-0-12-397918-6.00001-X. Methods Enzymol. 2014. PMID: 25399640 Free PMC article. Review.
Cited by
-
Back From the Dead: The Atypical Kinase Activity of a Pseudokinase Regulator of Cation Fluxes During Inducible Immunity.Front Plant Sci. 2022 Aug 11;13:931324. doi: 10.3389/fpls.2022.931324. eCollection 2022. Front Plant Sci. 2022. PMID: 36035673 Free PMC article.
-
The molecular basis of the Caskin1 and Mint1 interaction with CASK.J Mol Biol. 2011 Sep 9;412(1):3-13. doi: 10.1016/j.jmb.2011.07.005. Epub 2011 Jul 12. J Mol Biol. 2011. PMID: 21763699 Free PMC article.
-
Two microcephaly-associated novel missense mutations in CASK specifically disrupt the CASK-neurexin interaction.Hum Genet. 2018 Mar;137(3):231-246. doi: 10.1007/s00439-018-1874-3. Epub 2018 Feb 9. Hum Genet. 2018. PMID: 29426960 Free PMC article.
-
Optic Nerve Hypoplasia Is a Pervasive Subcortical Pathology of Visual System in Neonates.Invest Ophthalmol Vis Sci. 2017 Oct 1;58(12):5485-5496. doi: 10.1167/iovs.17-22399. Invest Ophthalmol Vis Sci. 2017. PMID: 29067402 Free PMC article.
-
Neuron-specific protein interactions of Drosophila CASK-β are revealed by mass spectrometry.Front Mol Neurosci. 2014 Jun 30;7:58. doi: 10.3389/fnmol.2014.00058. eCollection 2014. Front Mol Neurosci. 2014. PMID: 25071438 Free PMC article.
References
-
- Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–34. - PubMed
-
- Noble ME, Endicott JA, Johnson LN. Protein kinase inhibitors: insights into drug design from structure. Science. 2004;303:1800–5. - PubMed
-
- Huse M, Kuriyan J. The conformational plasticity of protein kinases. Cell. 2002;109:275–82. - PubMed
-
- Hanks SK, Quinn AM, Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988;241:42–52. - PubMed
-
- Taylor SS, Radzio-Andzelm E. Three protein kinase structures define a common motif. Structure. 1994;2:345–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

