Tissue-specific variation of Ube3a protein expression in rodents and in a mouse model of Angelman syndrome
- PMID: 20423730
- PMCID: PMC2922926
- DOI: 10.1016/j.nbd.2010.04.012
Tissue-specific variation of Ube3a protein expression in rodents and in a mouse model of Angelman syndrome
Abstract
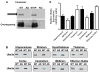
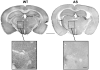
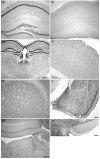
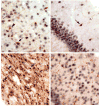
Angelman syndrome (AS) is a neurogenetic disorder caused by loss of maternal UBE3A expression or mutation-induced dysfunction of its protein product, the E3 ubiquitin-protein ligase, UBE3A. In humans and rodents, UBE3A/Ube3a transcript is maternally imprinted in several brain regions, but the distribution of native UBE3A/Ube3a(1) protein expression has not been comprehensively examined. To address this, we systematically evaluated Ube3a expression in the brain and peripheral tissues of wild-type (WT) and Ube3a maternal knockout mice (AS mice). Immunoblot and immunohistochemical analyses revealed a marked loss of Ube3a protein in hippocampus, hypothalamus, olfactory bulb, cerebral cortex, striatum, thalamus, midbrain, and cerebellum in AS mice relative to WT littermates. Also, Ube3a expression in heart and liver of AS mice showed greater than the predicted 50% reduction relative to WT mice. Co-localization studies showed Ube3a expression to be primarily neuronal in all brain regions and present in GABAergic interneurons as well as principal neurons. These findings suggest that neuronal function throughout the brain is compromised in AS.
Figures

Similar articles
-
Imprinting effects of UBE3A loss on synaptic gene networks and Wnt signaling pathways.Hum Mol Genet. 2019 Nov 15;28(22):3842-3852. doi: 10.1093/hmg/ddz221. Hum Mol Genet. 2019. PMID: 31625566 Free PMC article.
-
Loss of dopaminergic neurons and resulting behavioural deficits in mouse model of Angelman syndrome.Neurobiol Dis. 2010 Dec;40(3):586-92. doi: 10.1016/j.nbd.2010.08.002. Epub 2010 Aug 6. Neurobiol Dis. 2010. PMID: 20696245
-
Imprinted expression of the murine Angelman syndrome gene, Ube3a, in hippocampal and Purkinje neurons.Nat Genet. 1997 Sep;17(1):75-8. doi: 10.1038/ng0997-75. Nat Genet. 1997. PMID: 9288101
-
Towards an understanding of Angelman syndrome in mice studies.J Neurosci Res. 2020 Jun;98(6):1162-1173. doi: 10.1002/jnr.24576. Epub 2019 Dec 22. J Neurosci Res. 2020. PMID: 31867793 Review.
-
Angelman syndrome: insights into genomic imprinting and neurodevelopmental phenotypes.Trends Neurosci. 2011 Jun;34(6):293-303. doi: 10.1016/j.tins.2011.04.001. Epub 2011 May 17. Trends Neurosci. 2011. PMID: 21592595 Free PMC article. Review.
Cited by
-
Angelman Syndrome.Neurotherapeutics. 2015 Jul;12(3):641-50. doi: 10.1007/s13311-015-0361-y. Neurotherapeutics. 2015. PMID: 26040994 Free PMC article. Review.
-
Spatial and temporal silencing of the human maternal UBE3A gene.Eur J Paediatr Neurol. 2012 Nov;16(6):587-91. doi: 10.1016/j.ejpn.2012.03.006. Epub 2012 May 3. Eur J Paediatr Neurol. 2012. PMID: 22560727 Free PMC article.
-
Understanding the pathogenesis of Angelman syndrome through animal models.Neural Plast. 2012;2012:710943. doi: 10.1155/2012/710943. Epub 2012 Jul 8. Neural Plast. 2012. PMID: 22830052 Free PMC article. Review.
-
The Autism and Angelman Syndrome Protein Ube3A/E6AP: The Gene, E3 Ligase Ubiquitination Targets and Neurobiological Functions.Front Mol Neurosci. 2019 Apr 30;12:109. doi: 10.3389/fnmol.2019.00109. eCollection 2019. Front Mol Neurosci. 2019. PMID: 31114479 Free PMC article. Review.
-
From UBE3A to Angelman syndrome: a substrate perspective.Front Neurosci. 2015 Sep 15;9:322. doi: 10.3389/fnins.2015.00322. eCollection 2015. Front Neurosci. 2015. PMID: 26441497 Free PMC article. Review.
References
-
- Albrecht U, Sutcliffe JS, Cattanach BM, Beechey CV, Armstrong D, Eichele G, Beaudet AL. Imprinted expression of the murine Angelman syndrome gene, Ube3a, in hippocampal and Purkinje neurons. Nat Genet. 1997;17:75–78. - PubMed
-
- Beckung E, Steffenburg S, Kyllerman M. Motor impairments, neurological signs, and developmental level in individuals with Angelman syndrome. Dev Med Child Neurol. 2004;46:239–243. - PubMed
-
- Bubser M, Deutch AY. Stress induces Fos expression in neurons of the thalamic paraventricular nucleus that innervate limbic forebrain sites. Synapse. 1999;32:13–22. - PubMed
-
- Cattanach BM, Barr JA, Beechey CV, Martin J, Noebels J, Jones J. A candidate model for Angelman syndrome in the mouse. Mamm Genome. 1997;8:472–478. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

