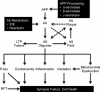
Current therapeutic targets for the treatment of Alzheimer's disease
- PMID: 20420492
- PMCID: PMC4140224
- DOI: 10.1586/ern.10.29
Current therapeutic targets for the treatment of Alzheimer's disease
Abstract
Alzheimer's disease is a progressive neurodegenerative disease for which no cure exists. There is a substantial need for new therapies that offer improved symptomatic benefit and disease-slowing capabilities. In recent decades there has been substantial progress in understanding the molecular and cellular changes associated with Alzheimer's disease pathology. This has resulted in identification of a large number of new drug targets. These targets include, but are not limited to, therapies that aim to prevent production of or remove the amyloid-beta protein that accumulates in neuritic plaques; to prevent the hyperphosphorylation and aggregation into paired helical filaments of the microtubule-associated protein tau; and to keep neurons alive and functioning normally in the face of these pathologic challenges. We provide a review of these targets for drug development.
Figures
Similar articles
-
Beneficial effect of human anti-amyloid-beta active immunization on neurite morphology and tau pathology.Brain. 2010 May;133(Pt 5):1312-27. doi: 10.1093/brain/awq056. Epub 2010 Mar 31. Brain. 2010. PMID: 20360050 Free PMC article. Clinical Trial.
-
Mechanism-based treatments for Alzheimer's disease.Dialogues Clin Neurosci. 2009;11(2):159-69. doi: 10.31887/DCNS.2009.11.2/pdavies. Dialogues Clin Neurosci. 2009. PMID: 19585951 Free PMC article. Review.
-
Pharmacotherapeutic targets in Alzheimer's disease.J Cell Mol Med. 2009 Jan;13(1):61-86. doi: 10.1111/j.1582-4934.2008.00595.x. Epub 2008 Nov 18. J Cell Mol Med. 2009. PMID: 19040415 Free PMC article. Review.
-
Beyond the neurotransmitter-focused approach in treating Alzheimer's disease: drugs targeting beta-amyloid and tau protein.Aging Clin Exp Res. 2009 Dec;21(6):386-406. doi: 10.1007/BF03327445. Aging Clin Exp Res. 2009. PMID: 20154508 Review.
-
Treatments for Alzheimer's disease emerge.Science. 2021 Aug 6;373(6555):624-626. doi: 10.1126/science.abi6401. Science. 2021. PMID: 34353940 No abstract available.
Cited by
-
Nasal delivery of neurotherapeutics via nanocarriers: Facets, aspects, and prospects.Front Pharmacol. 2022 Sep 13;13:979682. doi: 10.3389/fphar.2022.979682. eCollection 2022. Front Pharmacol. 2022. PMID: 36176429 Free PMC article. Review.
-
Dendrimers-Novel Therapeutic Approaches for Alzheimer's Disease.Biomedicines. 2024 Aug 20;12(8):1899. doi: 10.3390/biomedicines12081899. Biomedicines. 2024. PMID: 39200363 Free PMC article. Review.
-
Novel drug targets based on metallobiology of Alzheimer's disease.Expert Opin Ther Targets. 2010 Nov;14(11):1177-97. doi: 10.1517/14728222.2010.525352. Expert Opin Ther Targets. 2010. PMID: 20942746 Free PMC article. Review.
-
Future Therapeutic Perspectives into the Alzheimer's Disease Targeting the Oxidative Stress Hypothesis.Molecules. 2019 Dec 3;24(23):4410. doi: 10.3390/molecules24234410. Molecules. 2019. PMID: 31816853 Free PMC article. Review.
-
New compounds from heterocyclic amines scaffold with multitarget inhibitory activity on Aβ aggregation, AChE, and BACE1 in the Alzheimer disease.PLoS One. 2022 Jun 3;17(6):e0269129. doi: 10.1371/journal.pone.0269129. eCollection 2022. PLoS One. 2022. PMID: 35657793 Free PMC article.
References
-
- Rogers SL, Doody RS, Mohs RC, Friedhoff LT. Arch Intern Med. 9. Vol. 158. Donepezil Study Group; 1998. Donepezil improves cognition and global function in Alzheimer disease: a 15-week, double-blind, placebo-controlled study. pp. 1021–1031. - PubMed
-
- Rogers SL, Doody RS, Pratt RD, Ieni JR. Long-term efficacy and safety of donepezil in the treatment of Alzheimer's disease: final analysis of a US multicentre open-label study. Eur Neuropsychopharmacol. 2000;10(3):195–203. - PubMed
-
- Tariot PN, Cummings JL, Katz IR, et al. A randomized, double-blind, placebo-controlled study of the efficacy and safety of donepezil in patients with Alzheimer's disease in the nursing home setting. J Am Geriatr Soc. 2001;49(12):1590–1599. - PubMed
-
- Tariot PN, Solomon PR, Morris JC, Kershaw P, Lilienfeld S, Ding C. Neurology. 12. Vol. 54. The Galantamine USA-10 Study Group; 2000. A 5-month, randomized, placebo-controlled trial of galantamine in AD. pp. 2269–2276. - PubMed