Cytotoxic T lymphocytes established by seasonal human influenza cross-react against 2009 pandemic H1N1 influenza virus
- PMID: 20410263
- PMCID: PMC2903266
- DOI: 10.1128/JVI.00519-10
Cytotoxic T lymphocytes established by seasonal human influenza cross-react against 2009 pandemic H1N1 influenza virus
Abstract
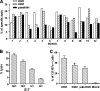
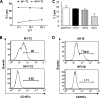
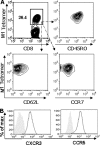
While few children and young adults have cross-protective antibodies to the pandemic H1N1 2009 (pdmH1N1) virus, the illness remains mild. The biological reasons for these epidemiological observations are unclear. In this study, we demonstrate that the bulk memory cytotoxic T lymphocytes (CTLs) established by seasonal influenza viruses from healthy individuals who have not been exposed to pdmH1N1 can directly lyse pdmH1N1-infected target cells and produce gamma interferon (IFN-gamma) and tumor necrosis factor alpha (TNF-alpha). Using influenza A virus matrix protein 1 (M1(58-66)) epitope-specific CTLs isolated from healthy HLA-A2(+) individuals, we further found that M1(58-66) epitope-specific CTLs efficiently killed both M1(58-66) peptide-pulsed and pdmH1N1-infected target cells ex vivo. These M1(58-66)-specific CTLs showed an effector memory phenotype and expressed CXCR3 and CCR5 chemokine receptors. Of 94 influenza A virus CD8 T-cell epitopes obtained from the Immune Epitope Database (IEDB), 17 epitopes are conserved in pdmH1N1, and more than half of these conserved epitopes are derived from M1 protein. In addition, 65% (11/17) of these epitopes were 100% conserved in seasonal influenza vaccine H1N1 strains during the last 20 years. Importantly, seasonal influenza vaccination could expand the functional M1(58-66) epitope-specific CTLs in 20% (4/20) of HLA-A2(+) individuals. Our results indicated that memory CTLs established by seasonal influenza A viruses or vaccines had cross-reactivity against pdmH1N1. These might explain, at least in part, the unexpected mild pdmH1N1 illness in the community and also might provide some valuable insights for the future design of broadly protective vaccines to prevent influenza, especially pandemic influenza.
Figures
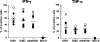


Similar articles
-
Human cytotoxic T lymphocytes directed to seasonal influenza A viruses cross-react with the newly emerging H7N9 virus.J Virol. 2014 Feb;88(3):1684-93. doi: 10.1128/JVI.02843-13. Epub 2013 Nov 20. J Virol. 2014. PMID: 24257602 Free PMC article.
-
Broadly Protective CD8+ T Cell Immunity to Highly Conserved Epitopes Elicited by Heat Shock Protein gp96-Adjuvanted Influenza Monovalent Split Vaccine.J Virol. 2021 May 24;95(12):e00507-21. doi: 10.1128/JVI.00507-21. Print 2021 May 24. J Virol. 2021. PMID: 33827939 Free PMC article.
-
Variation at Extra-epitopic Amino Acid Residues Influences Suppression of Influenza Virus Replication by M158-66 Epitope-Specific CD8+ T Lymphocytes.J Virol. 2018 May 14;92(11):e00232-18. doi: 10.1128/JVI.00232-18. Print 2018 Jun 1. J Virol. 2018. PMID: 29593036 Free PMC article.
-
Recalling the Future: Immunological Memory Toward Unpredictable Influenza Viruses.Front Immunol. 2019 Jul 2;10:1400. doi: 10.3389/fimmu.2019.01400. eCollection 2019. Front Immunol. 2019. PMID: 31312199 Free PMC article. Review.
-
Induction of virus-specific cytotoxic T lymphocytes as a basis for the development of broadly protective influenza vaccines.J Biomed Biotechnol. 2011;2011:939860. doi: 10.1155/2011/939860. Epub 2011 Oct 5. J Biomed Biotechnol. 2011. PMID: 22007149 Free PMC article. Review.
Cited by
-
Homologous peptides derived from influenza A, B and C viruses induce variable CD8+ T cell responses with cross-reactive potential.Clin Transl Immunology. 2022 Oct 19;11(10):e1422. doi: 10.1002/cti2.1422. eCollection 2022. Clin Transl Immunology. 2022. PMID: 36275878 Free PMC article.
-
The role of cell-mediated immunity against influenza and its implications for vaccine evaluation.Front Immunol. 2022 Aug 16;13:959379. doi: 10.3389/fimmu.2022.959379. eCollection 2022. Front Immunol. 2022. PMID: 36052083 Free PMC article. Review.
-
Immunopathogenesis of 2009 pandemic influenza.Enferm Infecc Microbiol Clin. 2012 Oct;30 Suppl 4:18-24. doi: 10.1016/S0213-005X(12)70100-3. Enferm Infecc Microbiol Clin. 2012. PMID: 23116788 Free PMC article. Review.
-
Immunological assessment of influenza vaccines and immune correlates of protection.Expert Rev Vaccines. 2013 May;12(5):519-36. doi: 10.1586/erv.13.35. Expert Rev Vaccines. 2013. PMID: 23659300 Free PMC article. Review.
-
Seasonal influenza infection and live vaccine prime for a response to the 2009 pandemic H1N1 vaccine.Proc Natl Acad Sci U S A. 2011 Jan 18;108(3):1140-5. doi: 10.1073/pnas.1009908108. Epub 2011 Jan 3. Proc Natl Acad Sci U S A. 2011. PMID: 21199945 Free PMC article.
References
-
- Alter, G., J. M. Malenfant, and M. Altfeld. 2004. CD107a as a functional marker for the identification of natural killer cell activity. J. Immunol. Methods 294:15-22. - PubMed
-
- Boon, A. C., G. de Mutsert, D. van Baarle, D. J. Smith, A. S. Lapedes, R. A. Fouchier, K. Sintnicolaas, A. D. Osterhaus, and G. F. Rimmelzwaan. 2004. Recognition of homo- and heterosubtypic variants of influenza A viruses by human CD8+ T lymphocytes. J. Immunol. 172:2453-2460. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

