Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes
- PMID: 20404917
- PMCID: PMC2852405
- DOI: 10.1371/journal.pone.0010074
Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes
Abstract
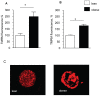
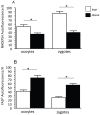
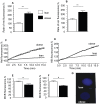
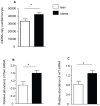
The negative impact of obesity on reproductive success is well documented but the stages at which development of the conceptus is compromised and the mechanisms responsible for the developmental failure still remain unclear. Recent findings suggest that mitochondria may be a contributing factor. However to date no studies have directly addressed the consequences of maternal obesity on mitochondria in early embryogenesis.Using an established murine model of maternal diet induced obesity and a live cell dynamic fluorescence imaging techniques coupled with molecular biology we have investigated the underlying mechanisms of obesity-induced reduced fertility. Our study is the first to show that maternal obesity prior to conception is associated with altered mitochondria in mouse oocytes and zygotes. Specifically, maternal diet-induced obesity in mice led to an increase in mitochondrial potential, mitochondrial DNA content and biogenesis. Generation of reactive oxygen species (ROS) was raised while glutathione was depleted and the redox state became more oxidised, suggestive of oxidative stress. These altered mitochondrial properties were associated with significant developmental impairment as shown by the increased number of obese mothers who failed to support blastocyst formation compared to lean dams. We propose that compromised oocyte and early embryo mitochondrial metabolism, resulting from excessive nutrient exposure prior to and during conception, may underlie poor reproductive outcomes frequently reported in obese women.
Conflict of interest statement
Figures
Similar articles
-
Mitochondria-targeted therapy rescues development and quality of embryos derived from oocytes matured under oxidative stress conditions: a bovine in vitro model.Hum Reprod. 2019 Oct 2;34(10):1984-1998. doi: 10.1093/humrep/dez161. Hum Reprod. 2019. PMID: 31625574
-
Maternal supply of omega-3 polyunsaturated fatty acids alter mechanisms involved in oocyte and early embryo development in the mouse.Am J Physiol Endocrinol Metab. 2008 Feb;294(2):E425-34. doi: 10.1152/ajpendo.00409.2007. Epub 2007 Dec 11. Am J Physiol Endocrinol Metab. 2008. PMID: 18073322
-
The effect of maternal high-fat/high-sugar diet on offspring oocytes and early embryo development.Mol Hum Reprod. 2019 Nov 30;25(11):717-728. doi: 10.1093/molehr/gaz049. Mol Hum Reprod. 2019. PMID: 31588490 Free PMC article.
-
Maternal obesity, infertility and mitochondrial dysfunction: potential mechanisms emerging from mouse model systems.Mol Hum Reprod. 2013 Aug;19(8):486-94. doi: 10.1093/molehr/gat026. Epub 2013 Apr 23. Mol Hum Reprod. 2013. PMID: 23612738 Free PMC article. Review.
-
Symposium review: Reduction in oocyte developmental competence by stress is associated with alterations in mitochondrial function.J Dairy Sci. 2018 Apr;101(4):3642-3654. doi: 10.3168/jds.2017-13389. Epub 2018 Feb 1. J Dairy Sci. 2018. PMID: 29395145 Review.
Cited by
-
Endoplasmic reticulum (ER) stress in cumulus-oocyte complexes impairs pentraxin-3 secretion, mitochondrial membrane potential (DeltaPsi m), and embryo development.Mol Endocrinol. 2012 Apr;26(4):562-73. doi: 10.1210/me.2011-1362. Epub 2012 Mar 1. Mol Endocrinol. 2012. PMID: 22383462 Free PMC article.
-
Regulation of [Ca2+]i oscillations and mitochondrial activity by various calcium transporters in mouse oocytes.Reprod Biol Endocrinol. 2020 Aug 15;18(1):87. doi: 10.1186/s12958-020-00643-7. Reprod Biol Endocrinol. 2020. PMID: 32799904 Free PMC article.
-
Carnitines as Mitochondrial Modulators of Oocyte and Embryo Bioenergetics.Antioxidants (Basel). 2022 Apr 8;11(4):745. doi: 10.3390/antiox11040745. Antioxidants (Basel). 2022. PMID: 35453430 Free PMC article. Review.
-
Glutathione deficiency decreases lipid droplet stores and increases reactive oxygen species in mouse oocytes†.Biol Reprod. 2022 Jun 13;106(6):1218-1231. doi: 10.1093/biolre/ioac032. Biol Reprod. 2022. PMID: 35238901 Free PMC article.
-
Early programming of reproductive health and fertility: novel neuroendocrine mechanisms and implications in reproductive medicine.Hum Reprod Update. 2022 May 2;28(3):346-375. doi: 10.1093/humupd/dmac005. Hum Reprod Update. 2022. PMID: 35187579 Free PMC article. Review.
References
-
- Baker P, Balen A, Poston L, Sattar N. Obesity and Reproductive health: RCOG Press. 2007. 286
-
- Metwally M, Li TC, Ledger WL. The impact of obesity on female reproductive function. Obes Rev. 2007;8:515–523. - PubMed
-
- Maheshwari A, Stofberg L, Bhattacharya S. Effect of overweight and obesity on assisted reproductive technology–a systematic review. Hum Reprod Update. 2007;13:433–444. - PubMed
-
- Robker RL. Evidence that obesity alters the quality of oocytes and embryos. Pathophysiology. 2008;15:115–121. - PubMed
Publication types
MeSH terms
Grants and funding
- BB/F007450/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- F007450/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/C518273/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- G18784/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- G0100558/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

