Vaccination with synthetic analog peptides derived from WT1 oncoprotein induces T-cell responses in patients with complete remission from acute myeloid leukemia
- PMID: 20400682
- PMCID: PMC2910606
- DOI: 10.1182/blood-2009-10-250993
Vaccination with synthetic analog peptides derived from WT1 oncoprotein induces T-cell responses in patients with complete remission from acute myeloid leukemia
Abstract
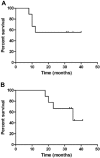
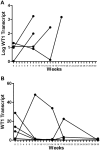
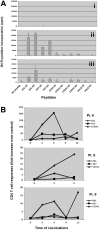
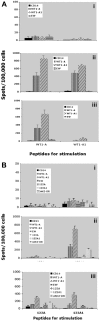
A pilot study was undertaken to assess the safety, activity, and immunogenicity of a polyvalent Wilms tumor gene 1 (WT1) peptide vaccine in patients with acute myeloid leukemia in complete remission but with molecular evidence of WT1 transcript. Patients received 6 vaccinations with 4 WT1 peptides (200 microg each) plus immune adjuvants over 12 weeks. Immune responses were evaluated by delayed-type hypersensitivity, CD4+ T-cell proliferation, CD3+ T-cell interferon-gamma release, and WT1 peptide tetramer staining. Of the 9 evaluable patients, 7 completed 6 vaccinations and WT1-specific T-cell responses were noted in 7 of 8 patients. Three patients who were HLA-A0201-positive showed significant increase in interferon-gamma-secreting cells and frequency of WT1 tetramer-positive CD8+ T cells. Three patients developed a delayed hypersensitivity reaction after vaccination. Definite related toxicities were minimal. With a mean follow-up of 30 plus or minus 8 months after diagnosis, median disease-free survival has not been reached. These preliminary data suggest that this polyvalent WT1 peptide vaccine can be administered safely to patients with a resulting immune response. Further studies are needed to establish the role of vaccination as viable postremission therapy for acute myeloid leukemia.
Trial registration: ClinicalTrials.gov NCT00398138.
Figures

Similar articles
-
WT1 vaccination in AML and MDS: A pilot trial with synthetic analog peptides.Am J Hematol. 2015 Jul;90(7):602-7. doi: 10.1002/ajh.24014. Epub 2015 May 3. Am J Hematol. 2015. PMID: 25802083 Free PMC article. Clinical Trial.
-
Phase 2 trial of a multivalent WT1 peptide vaccine (galinpepimut-S) in acute myeloid leukemia.Blood Adv. 2018 Feb 13;2(3):224-234. doi: 10.1182/bloodadvances.2017014175. Blood Adv. 2018. PMID: 29386195 Free PMC article. Clinical Trial.
-
Induction of complete and molecular remissions in acute myeloid leukemia by Wilms' tumor 1 antigen-targeted dendritic cell vaccination.Proc Natl Acad Sci U S A. 2010 Aug 3;107(31):13824-9. doi: 10.1073/pnas.1008051107. Epub 2010 Jul 14. Proc Natl Acad Sci U S A. 2010. PMID: 20631300 Free PMC article. Clinical Trial.
-
WT1 peptide cancer vaccine for patients with hematopoietic malignancies and solid cancers.ScientificWorldJournal. 2007 May 29;7:649-65. doi: 10.1100/tsw.2007.119. ScientificWorldJournal. 2007. PMID: 17619750 Free PMC article. Review.
-
WT1 as a novel target antigen for cancer immunotherapy.Curr Cancer Drug Targets. 2002 Mar;2(1):45-54. doi: 10.2174/1568009023334088. Curr Cancer Drug Targets. 2002. PMID: 12188920 Review.
Cited by
-
Lentivirus-induced dendritic cells for immunization against high-risk WT1(+) acute myeloid leukemia.Hum Gene Ther. 2013 Feb;24(2):220-37. doi: 10.1089/hum.2012.128. Hum Gene Ther. 2013. PMID: 23311414 Free PMC article.
-
Autologous transplantation gives encouraging results for young adults with favorable-risk acute myeloid leukemia, but is not improved with gemtuzumab ozogamicin.Blood. 2011 May 19;117(20):5306-13. doi: 10.1182/blood-2010-09-309229. Epub 2011 Mar 17. Blood. 2011. PMID: 21415269 Free PMC article. Clinical Trial.
-
An immunogenic WT1-derived peptide that induces T cell response in the context of HLA-A*02:01 and HLA-A*24:02 molecules.Oncoimmunology. 2016 Dec 7;6(2):e1252895. doi: 10.1080/2162402X.2016.1252895. eCollection 2017. Oncoimmunology. 2016. PMID: 28344864 Free PMC article.
-
New Horizons in Immunology and Immunotherapy of Acute Leukemias and Related Disorders.Cancers (Basel). 2023 Apr 23;15(9):2422. doi: 10.3390/cancers15092422. Cancers (Basel). 2023. PMID: 37173889 Free PMC article.
-
Targeting of the WT191-138 fragment to human dendritic cells improves leukemia-specific T-cell responses providing an alternative approach to WT1-based vaccination.Cancer Immunol Immunother. 2017 Mar;66(3):319-332. doi: 10.1007/s00262-016-1938-y. Epub 2016 Nov 28. Cancer Immunol Immunother. 2017. PMID: 27896368 Free PMC article.
References
-
- Frattini MG, Maslak PG. Strategy for incorporating molecular and cytogenetic markers into acute myeloid leukemia therapy. J Natl Compr Canc Netw. 2008;6(10):995–1002. - PubMed
-
- Virappane P, Gale R, Hills R, et al. Mutation of the Wilms' tumor 1 gene is a poor prognostic factor associated with chemotherapy resistance in normal karyotype acute myeloid leukemia: the United Kingdom Medical Research Council Adult Leukaemia Working Party. J Clin Oncol. 2008;26(33):5429–5435. - PubMed
-
- Scheibenbogen C, Letsch A, Thiel E, et al. CD8 T-cell responses to Wilms tumor gene product WT1 and proteinase 3 in patients with acute myeloid leukemia. Blood. 2002;100(6):2132–2137. - PubMed
-
- Rezvani K, Grube M, Brenchley JM, et al. Functional leukemia-associated antigen-specific memory CD8+ T cells exist in healthy individuals and in patients with chronic myelogenous leukemia before and after stem cell transplantation. Blood. 2003;102(8):2892–2900. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

