Arrangement of photosystem II and ATP synthase in chloroplast membranes of spinach and pea
- PMID: 20388855
- PMCID: PMC2879734
- DOI: 10.1105/tpc.109.071431
Arrangement of photosystem II and ATP synthase in chloroplast membranes of spinach and pea
Abstract
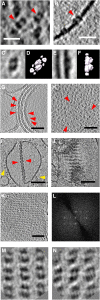
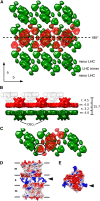
We used cryoelectron tomography to reveal the arrangements of photosystem II (PSII) and ATP synthase in vitreous sections of intact chloroplasts and plunge-frozen suspensions of isolated thylakoid membranes. We found that stroma and grana thylakoids are connected at the grana margins by staggered lamellar membrane protrusions. The stacking repeat of grana membranes in frozen-hydrated chloroplasts is 15.7 nm, with a 4.5-nm lumenal space and a 3.2-nm distance between the flat stromal surfaces. The chloroplast ATP synthase is confined to minimally curved regions at the grana end membranes and stroma lamellae, where it covers 20% of the surface area. In total, 85% of the ATP synthases are monomers and the remainder form random assemblies of two or more copies. Supercomplexes of PSII and light-harvesting complex II (LHCII) occasionally form ordered arrays in appressed grana thylakoids, whereas this order is lost in destacked membranes. In the ordered arrays, each membrane on either side of the stromal gap contains a two-dimensional crystal of supercomplexes, with the two lattices arranged such that PSII cores, LHCII trimers, and minor LHCs each face a complex of the same kind in the opposite membrane. Grana formation is likely to result from electrostatic interactions between these complexes across the stromal gap.
Figures



Similar articles
-
High-Light versus Low-Light: Effects on Paired Photosystem II Supercomplex Structural Rearrangement in Pea Plants.Int J Mol Sci. 2020 Nov 16;21(22):8643. doi: 10.3390/ijms21228643. Int J Mol Sci. 2020. PMID: 33207833 Free PMC article.
-
Correlation between spatial (3D) structure of pea and bean thylakoid membranes and arrangement of chlorophyll-protein complexes.BMC Plant Biol. 2012 May 25;12:72. doi: 10.1186/1471-2229-12-72. BMC Plant Biol. 2012. PMID: 22631450 Free PMC article.
-
Pea PSII-LHCII supercomplexes form pairs by making connections across the stromal gap.Sci Rep. 2017 Aug 30;7(1):10067. doi: 10.1038/s41598-017-10700-8. Sci Rep. 2017. PMID: 28855679 Free PMC article.
-
Supramolecular organization of thylakoid membrane proteins in green plants.Biochim Biophys Acta. 2005 Jan 7;1706(1-2):12-39. doi: 10.1016/j.bbabio.2004.09.009. Biochim Biophys Acta. 2005. PMID: 15620363 Review.
-
Atomic force microscopy studies of native photosynthetic membranes.Biochemistry. 2009 May 5;48(17):3679-98. doi: 10.1021/bi900045x. Biochemistry. 2009. PMID: 19265434 Review.
Cited by
-
Excitonic connectivity between photosystem II units: what is it, and how to measure it?Photosynth Res. 2013 Oct;116(2-3):189-214. doi: 10.1007/s11120-013-9863-9. Epub 2013 Jun 21. Photosynth Res. 2013. PMID: 23794168 Review.
-
Similarities and Differences in the Effects of Toxic Concentrations of Cadmium and Chromium on the Structure and Functions of Thylakoid Membranes in Chlorella variabilis.Front Plant Sci. 2020 Jul 7;11:1006. doi: 10.3389/fpls.2020.01006. eCollection 2020. Front Plant Sci. 2020. PMID: 32733513 Free PMC article.
-
Reply to: Is the debate over grana stacking formation finally solved?Nat Plants. 2021 Mar;7(3):279-281. doi: 10.1038/s41477-021-00881-6. Epub 2021 Mar 11. Nat Plants. 2021. PMID: 33707740 No abstract available.
-
The Structural and Spectral Features of Light-Harvesting Complex II Proteoliposomes Mimic Those of Native Thylakoid Membranes.J Phys Chem Lett. 2022 Jun 23;13(24):5683-5691. doi: 10.1021/acs.jpclett.2c01019. Epub 2022 Jun 16. J Phys Chem Lett. 2022. PMID: 35709359 Free PMC article.
-
Spatial arrangement of proteins in planar and curved membranes by PPM 3.0.Protein Sci. 2022 Jan;31(1):209-220. doi: 10.1002/pro.4219. Epub 2021 Nov 8. Protein Sci. 2022. PMID: 34716622 Free PMC article.
References
-
- Al-Amoudi A., Studer D., Dubochet J. (2005). Cutting artefacts and cutting process in vitreous sections for cryo-electron microscopy. J. Struct. Biol. 150: 109–121 - PubMed
-
- Albertsson P.-A. (1982). Interaction between the lumenal sides of the thylakoid membrane. FEBS Lett. 149: 186–190
-
- Allen J.F. (1992). Protein phosphorylation in regulation of photosynthesis. Biochim. Biophys. Acta 1098: 275–335 - PubMed
-
- Anderson J.M., Chow W.S., De Las Rivas J. (2008). Dynamic flexibility in the structure and function of photosystem II in higher plant thylakoid membranes: the grana enigma. Photosynth. Res. 98: 575–587 - PubMed
-
- Andersson B., Anderson J.M. (1980). Lateral heterogeneity in the distribution of chlorophyll-protein complexes of the thylakoid membranes of spinach chloroplasts. Biochim. Biophys. Acta 593: 427–440 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

