The creation and physiological relevance of divergent hydroxylation patterns in the flavonoid pathway
- PMID: 20386656
- PMCID: PMC2852856
- DOI: 10.3390/ijms11020595
The creation and physiological relevance of divergent hydroxylation patterns in the flavonoid pathway
Abstract
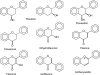
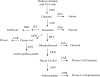
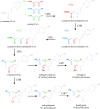
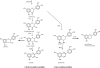
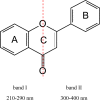
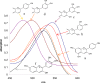
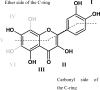
Flavonoids and biochemically-related chalcones are important secondary metabolites, which are ubiquitously present in plants and therefore also in human food. They fulfill a broad range of physiological functions in planta and there are numerous reports about their physiological relevance for humans. Flavonoids have in common a basic C(6)-C(3)-C(6) skeleton structure consisting of two aromatic rings (A and B) and a heterocyclic ring (C) containing one oxygen atom, whereas chalcones, as the intermediates in the formation of flavonoids, have not yet established the heterocyclic C-ring. Flavonoids are grouped into eight different classes, according to the oxidative status of the C-ring. The large number of divergent chalcones and flavonoid structures is from the extensive modification of the basic molecules. The hydroxylation pattern influences physiological properties such as light absorption and antioxidative activity, which is the base for many beneficial health effects of flavonoids. In some cases antiinfective properties are also effected.
Keywords: 2-oxoglutarate-Fe(II)-dependent dioxygenase; chalcone; cytochrome P450 dependent monooxygenase; flavonoid; hydroxylation; oxidoreductase; plant.
Figures
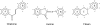
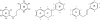
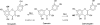

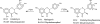


Similar articles
-
Regulation of vascular endothelial genes by dietary flavonoids: structure-expression relationship studies and the role of the transcription factor KLF-2.J Nutr Biochem. 2015 Mar;26(3):277-84. doi: 10.1016/j.jnutbio.2014.11.003. Epub 2014 Dec 9. J Nutr Biochem. 2015. PMID: 25542418
-
Effects of flavonoids on the release of reactive oxygen species by stimulated human neutrophils. Multivariate analysis of structure-activity relationships (SAR).Biochem Pharmacol. 1993 Oct 5;46(7):1257-71. doi: 10.1016/0006-2952(93)90476-d. Biochem Pharmacol. 1993. PMID: 8216378
-
Poplar MYB117 promotes anthocyanin synthesis and enhances flavonoid B-ring hydroxylation by up-regulating the flavonoid 3',5'-hydroxylase gene.J Exp Bot. 2021 May 4;72(10):3864-3880. doi: 10.1093/jxb/erab116. J Exp Bot. 2021. PMID: 33711094
-
Chemistry and biological activities of flavonoids: an overview.ScientificWorldJournal. 2013 Dec 29;2013:162750. doi: 10.1155/2013/162750. eCollection 2013. ScientificWorldJournal. 2013. PMID: 24470791 Free PMC article. Review.
-
Flavonoids as antioxidants in plants: location and functional significance.Plant Sci. 2012 Nov;196:67-76. doi: 10.1016/j.plantsci.2012.07.014. Epub 2012 Aug 11. Plant Sci. 2012. PMID: 23017900 Review.
Cited by
-
Molecular and Enzymatic Characterization of Flavonoid 3'-Hydroxylase of Malus × domestica.Plants (Basel). 2021 Sep 19;10(9):1956. doi: 10.3390/plants10091956. Plants (Basel). 2021. PMID: 34579488 Free PMC article.
-
The genotoxicity potential of luteolin is enhanced by CYP1A1 and CYP1A2 in human lymphoblastoid TK6 cells.Toxicol Lett. 2021 Jun 15;344:58-68. doi: 10.1016/j.toxlet.2021.03.006. Epub 2021 Mar 13. Toxicol Lett. 2021. PMID: 33727136 Free PMC article.
-
The Anti-Cancer Effect of Polyphenols against Breast Cancer and Cancer Stem Cells: Molecular Mechanisms.Nutrients. 2016 Sep 21;8(9):581. doi: 10.3390/nu8090581. Nutrients. 2016. PMID: 27657126 Free PMC article. Review.
-
Transcriptomic and Proteomic Analysis of Drought Stress Response in Opium Poppy Plants during the First Week of Germination.Plants (Basel). 2021 Sep 10;10(9):1878. doi: 10.3390/plants10091878. Plants (Basel). 2021. PMID: 34579414 Free PMC article.
-
A phylogenetic examination of the primary anthocyanin production pathway of the Plantae.Bot Stud. 2014 Dec;55(1):10. doi: 10.1186/1999-3110-55-10. Epub 2014 Jan 25. Bot Stud. 2014. PMID: 28510914 Free PMC article.
References
-
- Stafford HA. Flavonoid Metabolism. CRC Press; Boca Raton, FL, USA: 1990.
-
- Harborne JB, Baxter H. A Handbook of the Natural Flavonoids. Wiley; Chichester, UK: 1999.
-
- Forkmann G, Heller W. Biosynthesis of flavonoids. In: Barton D, Nakanishi K, Meth-Cohn O, editors. Comprehensive Natural Products Chemistry. Vol. 1. Elsevier; Oxford, UK: 1999. pp. 713–748.
-
- Harborne JB. Comparative Biochemistry of the Flavonoids. Academic Press; New York, NY, USA: 1967.
-
- Bohm B. The minor flavonoids. Flavonoids. 1994:387–440.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

