A potential role for adiponectin receptor 2 (AdipoR2) in the regulation of alcohol intake
- PMID: 20380822
- PMCID: PMC2906226
- DOI: 10.1016/j.brainres.2010.03.060
A potential role for adiponectin receptor 2 (AdipoR2) in the regulation of alcohol intake
Abstract
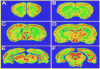
The anterior cingulate cortex (ACC) has been implicated in alcohol and drug addiction. We recently identified the small G protein K-ras as an alcohol-regulated gene in the ACC by gene expression analysis. We show here that the adiponectin receptor 2 (AdipoR2) was differentially regulated by alcohol in the ACC in a K-ras-dependent manner. Additionally, withdrawal-associated increased drinking was attenuated in AdipoR2 null mice. Intracellular recordings revealed that adiponectin increased the excitability of ACC neurons and that this effect was more pronounced during alcohol withdrawal, suggesting that AdipoR2 signaling may contribute to increased ACC activity. Altogether, the data implicate K-ras-regulated pathways involving AdipoR2 in the cellular and behavioral actions of alcohol that may contribute to overactivity of the ACC during withdrawal and excessive alcohol drinking.
Copyright 2010 Elsevier B.V. All rights reserved.
Figures
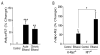
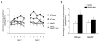
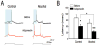
Similar articles
-
Genome-wide gene expression analysis identifies K-ras as a regulator of alcohol intake.Brain Res. 2010 Jun 21;1339:1-10. doi: 10.1016/j.brainres.2010.03.063. Epub 2010 Apr 10. Brain Res. 2010. PMID: 20388501 Free PMC article.
-
Adiponectin regulates contextual fear extinction and intrinsic excitability of dentate gyrus granule neurons through AdipoR2 receptors.Mol Psychiatry. 2017 Jul;22(7):1044-1055. doi: 10.1038/mp.2016.58. Epub 2016 May 3. Mol Psychiatry. 2017. PMID: 27137743 Free PMC article.
-
Effects of adrenal hormones on the expression of adiponectin and adiponectin receptors in adipose tissue, muscle and liver.Steroids. 2011 Nov;76(12):1260-7. doi: 10.1016/j.steroids.2011.06.004. Epub 2011 Jun 30. Steroids. 2011. PMID: 21745490
-
Adiponectin receptor binding proteins--recent advances in elucidating adiponectin signalling pathways.FEBS Lett. 2010 Oct 22;584(20):4280-6. doi: 10.1016/j.febslet.2010.09.035. Epub 2010 Sep 28. FEBS Lett. 2010. PMID: 20875820 Review.
-
The alcohol-preferring AA and alcohol-avoiding ANA rats: neurobiology of the regulation of alcohol drinking.Addict Biol. 2006 Sep;11(3-4):289-309. doi: 10.1111/j.1369-1600.2006.00037.x. Addict Biol. 2006. PMID: 16961760 Review.
Cited by
-
Escalated (Dependent) Oxycodone Self-Administration Is Associated with Cognitive Impairment and Transcriptional Evidence of Neurodegeneration in Human Immunodeficiency Virus (HIV) Transgenic Rats.Viruses. 2022 Mar 24;14(4):669. doi: 10.3390/v14040669. Viruses. 2022. PMID: 35458399 Free PMC article.
-
Protective effects of intracerebroventricular adiponectin against olfactory impairments in an amyloid β1-42 rat model.BMC Neurosci. 2021 Mar 2;22(1):14. doi: 10.1186/s12868-021-00620-9. BMC Neurosci. 2021. PMID: 33653273 Free PMC article.
-
Using genetically engineered animal models in the postgenomic era to understand gene function in alcoholism.Alcohol Res. 2012;34(3):282-91. Alcohol Res. 2012. PMID: 23134044 Free PMC article.
-
Adiponectin receptor signalling in the brain.Br J Pharmacol. 2012 Jan;165(2):313-27. doi: 10.1111/j.1476-5381.2011.01560.x. Br J Pharmacol. 2012. PMID: 21718299 Free PMC article. Review.
-
Increasing Adiponectin Signaling by Sub-Chronic AdipoRon Treatment Elicits Antidepressant- and Anxiolytic-Like Effects Independent of Changes in Hippocampal Plasticity.Biomedicines. 2023 Jan 18;11(2):249. doi: 10.3390/biomedicines11020249. Biomedicines. 2023. PMID: 36830788 Free PMC article.
References
-
- Acheson A, Robinson JL, Glahn DC, Lovallo WR, Fox PT. Differential activation of the anterior cingulate cortex and caudate nucleus during a gambling simulation in persons with a family history of alcoholism: studies from the Oklahoma Family Health Patterns Project. Drug Alcohol Depend. 2009;100:17–23. - PMC - PubMed
-
- Addolorato G, Leggio L, Hillemacher T, Kraus T, Jerlhag E, Bleich S. Hormones and drinking behaviour: new findings on ghrelin, insulin, leptin and volume-regulating hormones. An ESBRA Symposium report. Drug Alcohol Rev. 2009;28:160–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 AA006420/AA/NIAAA NIH HHS/United States
- AA01319/AA/NIAAA NIH HHS/United States
- AA006420/AA/NIAAA NIH HHS/United States
- AA017371/AA/NIAAA NIH HHS/United States
- R01 AA013191-05/AA/NIAAA NIH HHS/United States
- K99 DA023680/DA/NIDA NIH HHS/United States
- AA013523/AA/NIAAA NIH HHS/United States
- R01 AA013191/AA/NIAAA NIH HHS/United States
- R01 AA017371/AA/NIAAA NIH HHS/United States
- DA023680/DA/NIDA NIH HHS/United States
- R00 DA023680/DA/NIDA NIH HHS/United States
- DA027129-0109/DA/NIDA NIH HHS/United States
- U01 AA013523/AA/NIAAA NIH HHS/United States
- R01 AA017371-03/AA/NIAAA NIH HHS/United States
- R01 AA017371-03S1/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

