SIRT2 inhibition achieves neuroprotection by decreasing sterol biosynthesis
- PMID: 20378838
- PMCID: PMC2867924
- DOI: 10.1073/pnas.1002924107
SIRT2 inhibition achieves neuroprotection by decreasing sterol biosynthesis
Abstract
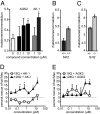
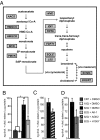
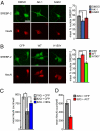
Huntington's disease (HD), an incurable neurodegenerative disorder, has a complex pathogenesis including protein aggregation and the dysregulation of neuronal transcription and metabolism. Here, we demonstrate that inhibition of sirtuin 2 (SIRT2) achieves neuroprotection in cellular and invertebrate models of HD. Genetic or pharmacologic inhibition of SIRT2 in a striatal neuron model of HD resulted in gene expression changes including significant down-regulation of RNAs responsible for sterol biosynthesis. Whereas mutant huntingtin fragments increased sterols in neuronal cells, SIRT2 inhibition reduced sterol levels via decreased nuclear trafficking of SREBP-2. Importantly, manipulation of sterol biosynthesis at the transcriptional level mimicked SIRT2 inhibition, demonstrating that the metabolic effects of SIRT2 inhibition are sufficient to diminish mutant huntingtin toxicity. These data identify SIRT2 inhibition as a promising avenue for HD therapy and elucidate a unique mechanism of SIRT2-inhibitor-mediated neuroprotection. Furthermore, the ascertainment of SIRT2's role in regulating cellular metabolism demonstrates a central function shared with other sirtuin proteins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Comment in
-
Neuroprotection and brain cholesterol biosynthesis in Huntington's disease.Proc Natl Acad Sci U S A. 2010 Sep 14;107(37):E143; author reply 144. doi: 10.1073/pnas.1006783107. Epub 2010 Aug 31. Proc Natl Acad Sci U S A. 2010. PMID: 20807740 Free PMC article. No abstract available.
Similar articles
-
The sirtuin 2 inhibitor AK-7 is neuroprotective in Huntington's disease mouse models.Cell Rep. 2012 Dec 27;2(6):1492-7. doi: 10.1016/j.celrep.2012.11.001. Epub 2012 Nov 29. Cell Rep. 2012. PMID: 23200855 Free PMC article.
-
SIRT2 ablation has no effect on tubulin acetylation in brain, cholesterol biosynthesis or the progression of Huntington's disease phenotypes in vivo.PLoS One. 2012;7(4):e34805. doi: 10.1371/journal.pone.0034805. Epub 2012 Apr 12. PLoS One. 2012. PMID: 22511966 Free PMC article.
-
SIRT2- and NRF2-Targeting Thiazole-Containing Compound with Therapeutic Activity in Huntington's Disease Models.Cell Chem Biol. 2016 Jul 21;23(7):849-861. doi: 10.1016/j.chembiol.2016.05.015. Epub 2016 Jul 14. Cell Chem Biol. 2016. PMID: 27427231
-
Review of the anti-inflammatory effect of SIRT1 and SIRT2 modulators on neurodegenerative diseases.Eur J Pharmacol. 2020 Jan 15;867:172847. doi: 10.1016/j.ejphar.2019.172847. Epub 2019 Dec 5. Eur J Pharmacol. 2020. PMID: 31812544 Review.
-
Sirtuins as Modifiers of Huntington's Disease (HD) Pathology.Prog Mol Biol Transl Sci. 2018;154:105-145. doi: 10.1016/bs.pmbts.2017.11.013. Epub 2017 Dec 27. Prog Mol Biol Transl Sci. 2018. PMID: 29413175 Review.
Cited by
-
Design and Evaluation of 3-(Benzylthio)benzamide Derivatives as Potent and Selective SIRT2 Inhibitors.ACS Med Chem Lett. 2015 Mar 26;6(5):607-11. doi: 10.1021/acsmedchemlett.5b00075. eCollection 2015 May 14. ACS Med Chem Lett. 2015. PMID: 26005542 Free PMC article.
-
Multiple Roles of SIRT2 in Regulating Physiological and Pathological Signal Transduction.Genet Res (Camb). 2022 Aug 29;2022:9282484. doi: 10.1155/2022/9282484. eCollection 2022. Genet Res (Camb). 2022. PMID: 36101744 Free PMC article. Review.
-
Recombinant NAD-dependent SIR-2 protein of Leishmania donovani: immunobiochemical characterization as a potential vaccine against visceral leishmaniasis.PLoS Negl Trop Dis. 2015 Mar 6;9(3):e0003557. doi: 10.1371/journal.pntd.0003557. eCollection 2015 Mar. PLoS Negl Trop Dis. 2015. PMID: 25745863 Free PMC article.
-
Sirtuin 1 activator SRT2104 protects Huntington's disease mice.Ann Clin Transl Neurol. 2014 Dec;1(12):1047-52. doi: 10.1002/acn3.135. Epub 2014 Oct 31. Ann Clin Transl Neurol. 2014. PMID: 25574479 Free PMC article.
-
Network organization of the huntingtin proteomic interactome in mammalian brain.Neuron. 2012 Jul 12;75(1):41-57. doi: 10.1016/j.neuron.2012.05.024. Neuron. 2012. PMID: 22794259 Free PMC article.
References
-
- The Huntington’s Disease Collaborative Research Group A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72:971–983. - PubMed
-
- Luthi-Carter R. Huntington’s and other polyglutamine diseases: Many effects of single gene mutations. Drug Discov Today Dis Mech. 2007;4:111–119.
-
- Mangiarini L, et al. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996;87:493–506. - PubMed
-
- Steffan JS, et al. Histone deacetylase inhibitors arrest polyglutamine-dependent neurodegeneration in Drosophila. Nature. 2001;413:739–743. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

