M1 protein allows Group A streptococcal survival in phagocyte extracellular traps through cathelicidin inhibition
- PMID: 20375578
- PMCID: PMC3241932
- DOI: 10.1159/000203645
M1 protein allows Group A streptococcal survival in phagocyte extracellular traps through cathelicidin inhibition
Abstract
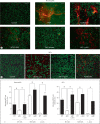
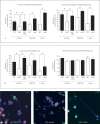
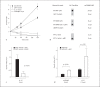
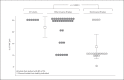
M1 protein contributes to Group A Streptococcus (GAS) systemic virulence by interfering with phagocytosis and through proinflammatory activities when released from the cell surface. Here we identify a novel role of M1 protein in the stimulation of neutrophil and mast cell extracellular trap formation, yet also subsequent survival of the pathogen within these DNA-based innate defense structures. Targeted mutagenesis and heterologous expression studies demonstrate M1 protein promotes resistance to the human cathelicidin antimicrobial peptide LL-37, an important effector of bacterial killing within such phagocyte extracellular traps. Studies with purified recombinant protein fragments mapped the inhibition of cathelicidin killing to the M1 hypervariable N-terminal domain. A survey of GAS clinical isolates found that strains from patients with necrotizing fasciitis or toxic shock syndrome were significantly more likely to be resistant to cathelicidin than GAS M types not associated with invasive disease; M1 isolates were uniformly resistant. We conclude increased resistance to host cathelicidin and killing within phagocyte extracellular traps contribute to the propensity of M1 GAS strains to produce invasive infections.
Copyright 2009 S. Karger AG, Basel.
Figures

Similar articles
-
Group A Streptococcal M1 Protein Sequesters Cathelicidin to Evade Innate Immune Killing.Cell Host Microbe. 2015 Oct 14;18(4):471-7. doi: 10.1016/j.chom.2015.09.004. Cell Host Microbe. 2015. PMID: 26468750 Free PMC article.
-
Streptococcal M1 Strikes by Neutralizing Cathelicidins.Cell Host Microbe. 2015 Oct 14;18(4):390-1. doi: 10.1016/j.chom.2015.10.002. Cell Host Microbe. 2015. PMID: 26468741
-
The fibrinogen-binding M1 protein reduces pharyngeal cell adherence and colonization phenotypes of M1T1 group A Streptococcus.J Biol Chem. 2014 Feb 7;289(6):3539-46. doi: 10.1074/jbc.M113.529537. Epub 2013 Dec 19. J Biol Chem. 2014. PMID: 24356958 Free PMC article.
-
Variation, Indispensability, and Masking in the M protein.Trends Microbiol. 2018 Feb;26(2):132-144. doi: 10.1016/j.tim.2017.08.002. Epub 2017 Aug 31. Trends Microbiol. 2018. PMID: 28867148 Free PMC article. Review.
-
Neutrophil extracellular traps: a strategic tactic to defeat pathogens with potential consequences for the host.J Innate Immun. 2009;1(3):176-80. doi: 10.1159/000203699. Epub 2009 Feb 20. J Innate Immun. 2009. PMID: 20375575 Free PMC article. Review.
Cited by
-
Neutrophil extracellular traps in physiology and pathology.Cent Eur J Immunol. 2014;39(1):116-21. doi: 10.5114/ceji.2014.42136. Epub 2014 Apr 17. Cent Eur J Immunol. 2014. PMID: 26155111 Free PMC article. Review.
-
Neutrophil Extracellular Traps and Liver Disease.Semin Liver Dis. 2020 May;40(2):171-179. doi: 10.1055/s-0039-3399562. Epub 2019 Nov 14. Semin Liver Dis. 2020. PMID: 31726473 Free PMC article. Review.
-
Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans.PLoS Pathog. 2009 Oct;5(10):e1000639. doi: 10.1371/journal.ppat.1000639. Epub 2009 Oct 30. PLoS Pathog. 2009. PMID: 19876394 Free PMC article.
-
Streptococcal inhibitor of complement promotes innate immune resistance phenotypes of invasive M1T1 group A Streptococcus.J Innate Immun. 2010;2(6):587-95. doi: 10.1159/000317672. Epub 2010 Sep 1. J Innate Immun. 2010. PMID: 20814187 Free PMC article.
-
Molecular insight into invasive group A streptococcal disease.Nat Rev Microbiol. 2011 Sep 16;9(10):724-36. doi: 10.1038/nrmicro2648. Nat Rev Microbiol. 2011. PMID: 21921933 Review.
References
-
- O'Grady KA, Kelpie L, Andrews RM, Curtis N, Nolan TM, Selvaraj G, Passmore JW, Oppedisano F, Carnie JA, Carapetis JR. The epidemiology of invasive group A streptococcal disease in Victoria, Australia. Med J Aust. 2007;186:565–569. - PubMed
-
- Darenberg J, Luca-Harari B, Jasir A, Sandgren A, Pettersson H, Schalen C, Norgren M, Romanus V, Norrby-Teglund A, Normark BH. Molecular and clinical characteristics of invasive group A streptococcal infection in Sweden. Clin Infect Dis. 2007;45:450–458. - PubMed
-
- O'Loughlin RE, Roberson A, Cieslak PR, Lynfield R, Gershman K, Craig A, Albanese BA, Farley MM, Barrett NL, Spina NL, Beall B, Harrison LH, Reingold A, van Beneden C. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000–2004. Clin Infect Dis. 2007;45:853–862. - PubMed
-
- Vlaminckx BJ, Mascini EM, Schellekens JF. Invasive Lancefield group A streptococcal infections in the Netherlands. Ned Tijdschr Geneeskd. 2007;151:1669–1673. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

