CD8+ T-cell recognition of human cytomegalovirus latency-associated determinant pUL138
- PMID: 20375220
- PMCID: PMC4091183
- DOI: 10.1099/vir.0.020982-0
CD8+ T-cell recognition of human cytomegalovirus latency-associated determinant pUL138
Abstract
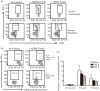
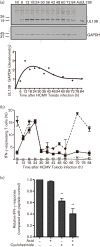
Recent studies have shown that long-term persistence of human cytomegalovirus (HCMV) in mononuclear cells of myeloid lineage is dependent on the UL138 open reading frame, which promotes latent infection. Although T-cell recognition of protein antigens from all stages of lytic HCMV infection is well established, it is not clear whether proteins expressed during latent HCMV infection can also be recognized. This study conducted an analysis of T-cell response towards proteins associated with HCMV latency. Ex vivo analysis of T cells from healthy virus carriers revealed a dominant CD8(+) T-cell response to the latency-associated pUL138 protein, which recognized a non-canonical 13 aa epitope in association with HLA-B*3501. These pUL138-specific T cells displayed a range of memory phenotypes that were in general less differentiated than that previously described in T cells specific for HCMV lytic antigens. Antigen-presentation assays revealed that endogenous pUL138 could be presented efficiently by HCMV-infected cells. However, T-cell recognition of pUL138 was dependent on newly synthesized protein, with little presentation from stable, long-lived protein. These data demonstrate that T cells targeting latency-associated protein products exist, although HCMV may limit the presentation of latent proteins, thereby restricting T-cell recognition of latently infected cells.
Figures


Similar articles
-
Human cytomegalovirus latency-associated proteins elicit immune-suppressive IL-10 producing CD4⁺ T cells.PLoS Pathog. 2013;9(10):e1003635. doi: 10.1371/journal.ppat.1003635. Epub 2013 Oct 10. PLoS Pathog. 2013. PMID: 24130479 Free PMC article. Clinical Trial.
-
Ex vivo profiling of CD8+-T-cell responses to human cytomegalovirus reveals broad and multispecific reactivities in healthy virus carriers.J Virol. 2003 May;77(9):5226-40. doi: 10.1128/jvi.77.9.5226-5240.2003. J Virol. 2003. PMID: 12692225 Free PMC article.
-
Characterization of a novel Golgi apparatus-localized latency determinant encoded by human cytomegalovirus.J Virol. 2009 Jun;83(11):5615-29. doi: 10.1128/JVI.01989-08. Epub 2009 Mar 18. J Virol. 2009. PMID: 19297488 Free PMC article.
-
Aspects of human cytomegalovirus latency and reactivation.Curr Top Microbiol Immunol. 2008;325:297-313. doi: 10.1007/978-3-540-77349-8_17. Curr Top Microbiol Immunol. 2008. PMID: 18637513 Review.
-
Generation, maintenance and tissue distribution of T cell responses to human cytomegalovirus in lytic and latent infection.Med Microbiol Immunol. 2019 Aug;208(3-4):375-389. doi: 10.1007/s00430-019-00598-6. Epub 2019 Mar 20. Med Microbiol Immunol. 2019. PMID: 30895366 Free PMC article. Review.
Cited by
-
Cytomegalovirus drives Vδ2neg γδ T cell inflation in many healthy virus carriers with increasing age.Clin Exp Immunol. 2014 Jun;176(3):418-28. doi: 10.1111/cei.12297. Clin Exp Immunol. 2014. PMID: 24547915 Free PMC article.
-
Cytomegalovirus-specific CD8+ T-cells are associated with a reduced incidence of early relapse after allogeneic stem cell transplantation.PLoS One. 2019 Mar 19;14(3):e0213739. doi: 10.1371/journal.pone.0213739. eCollection 2019. PLoS One. 2019. PMID: 30889204 Free PMC article.
-
Human cytomegalovirus manipulation of latently infected cells.Viruses. 2013 Nov 21;5(11):2803-24. doi: 10.3390/v5112803. Viruses. 2013. PMID: 24284875 Free PMC article. Review.
-
Human cytomegalovirus latency-associated proteins elicit immune-suppressive IL-10 producing CD4⁺ T cells.PLoS Pathog. 2013;9(10):e1003635. doi: 10.1371/journal.ppat.1003635. Epub 2013 Oct 10. PLoS Pathog. 2013. PMID: 24130479 Free PMC article. Clinical Trial.
-
Autophagy during viral infection - a double-edged sword.Nat Rev Microbiol. 2018 Jun;16(6):341-354. doi: 10.1038/s41579-018-0003-6. Nat Rev Microbiol. 2018. PMID: 29556036 Free PMC article. Review.
References
-
- Appay V., Dunbar P. R., Callan M., Klenerman P., Gillespie G. M., Papagno L., Ogg G. S., King A., Lechner F. & other authors (2002). Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med 8, 379–385 - PubMed
-
- Boeckh M., Nichols W. G., Papanicolaou G., Rubin R., Wingard J. R., Zaia J. (2003). Cytomegalovirus in hematopoietic stem cell transplant recipients: current status, known challenges, and future strategies. Biol Blood Marrow Transplant 9, 543–558 - PubMed
-
- Bolovan-Fritts C. A., Mocarski E. S., Wiedeman J. A. (1999). Peripheral blood CD14+ cells from healthy subjects carry a circular conformation of latent cytomegalovirus genome. Blood 93, 394–398 - PubMed
-
- Broers A. E., van Der Holt R., van Esser J. W., Gratama J. W., Henzen-Logmans S., Kuenen-Boumeester V., Lowenberg B., Cornelissen J. J. (2000). Increased transplant-related morbidity and mortality in CMV-seropositive patients despite highly effective prevention of CMV disease after allogeneic T-cell-depleted stem cell transplantation. Blood 95, 2240–2245 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

