Age-related molecular genetic changes of murine bone marrow mesenchymal stem cells
- PMID: 20374652
- PMCID: PMC2873471
- DOI: 10.1186/1471-2164-11-229
Age-related molecular genetic changes of murine bone marrow mesenchymal stem cells
Abstract
Background: Mesenchymal stem cells (MSC) are pluripotent cells, present in the bone marrow and other tissues that can differentiate into cells of all germ layers and may be involved in tissue maintenance and repair in adult organisms. Because of their plasticity and accessibility these cells are also prime candidates for regenerative medicine. The contribution of stem cell aging to organismal aging is under debate and one theory is that reparative processes deteriorate as a consequence of stem cell aging and/or decrease in number. Age has been linked with changes in osteogenic and adipogenic potential of MSCs.
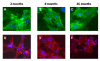
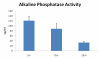
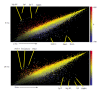
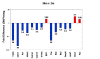
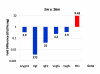
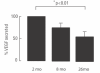
Results: Here we report on changes in global gene expression of cultured MSCs isolated from the bone marrow of mice at ages 2, 8, and 26-months. Microarray analyses revealed significant changes in the expression of more than 8000 genes with stage-specific changes of multiple differentiation, cell cycle and growth factor genes. Key markers of adipogenesis including lipoprotein lipase, FABP4, and Itm2a displayed age-dependent declines. Expression of the master cell cycle regulators p53 and p21 and growth factors HGF and VEGF also declined significantly at 26 months. These changes were evident despite multiple cell divisions in vitro after bone marrow isolation.
Conclusions: The results suggest that MSCs are subject to molecular genetic changes during aging that are conserved during passage in culture. These changes may affect the physiological functions and the potential of autologous MSCs for stem cell therapy.
Figures







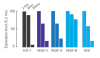
Similar articles
-
Tissue source determines the differentiation potentials of mesenchymal stem cells: a comparative study of human mesenchymal stem cells from bone marrow and adipose tissue.Stem Cell Res Ther. 2017 Dec 6;8(1):275. doi: 10.1186/s13287-017-0716-x. Stem Cell Res Ther. 2017. PMID: 29208029 Free PMC article.
-
Matrix-mediated retention of adipogenic differentiation potential by human adult bone marrow-derived mesenchymal stem cells during ex vivo expansion.Biomaterials. 2005 Nov;26(31):6167-75. doi: 10.1016/j.biomaterials.2005.03.024. Biomaterials. 2005. PMID: 15913765
-
Macrophage migration inhibitory factor confers resistance to senescence through CD74-dependent AMPK-FOXO3a signaling in mesenchymal stem cells.Stem Cell Res Ther. 2015 Apr 22;6(1):82. doi: 10.1186/s13287-015-0076-3. Stem Cell Res Ther. 2015. PMID: 25896286 Free PMC article.
-
Mesenchymal stem cells in the aging and osteoporotic population.Crit Rev Eukaryot Gene Expr. 2011;21(4):363-77. doi: 10.1615/critreveukargeneexpr.v21.i4.60. Crit Rev Eukaryot Gene Expr. 2011. PMID: 22181705 Review.
-
Bone marrow mesenchymal stem cells: Aging and tissue engineering applications to enhance bone healing.Biomaterials. 2019 May;203:96-110. doi: 10.1016/j.biomaterials.2018.06.026. Epub 2018 Jun 22. Biomaterials. 2019. PMID: 29980291 Free PMC article. Review.
Cited by
-
Crucial Role of Lamin A/C in the Migration and Differentiation of MSCs in Bone.Cells. 2020 May 26;9(6):1330. doi: 10.3390/cells9061330. Cells. 2020. PMID: 32466483 Free PMC article. Review.
-
MiR-126-HMGB1-HIF-1 Axis Regulates Endothelial Cell Inflammation during Exposure to Hypoxia-Acidosis.Dis Markers. 2021 Dec 21;2021:4933194. doi: 10.1155/2021/4933194. eCollection 2021. Dis Markers. 2021. PMID: 34970357 Free PMC article.
-
A dual role of HIF1α in regulating osteogenesis-angiogenesis coupling.Stem Cell Res Ther. 2022 Feb 5;13(1):59. doi: 10.1186/s13287-022-02742-1. Stem Cell Res Ther. 2022. PMID: 35123567 Free PMC article.
-
Human gingival mesenchymal stem cells retain their growth and immunomodulatory characteristics independent of donor age.Sci Adv. 2022 Jun 24;8(25):eabm6504. doi: 10.1126/sciadv.abm6504. Epub 2022 Jun 24. Sci Adv. 2022. PMID: 35749495 Free PMC article.
-
The therapeutic impact of human neonatal BMSC in a right ventricular pressure overload model in mice.Stem Cell Res Ther. 2020 Mar 2;11(1):96. doi: 10.1186/s13287-020-01593-y. Stem Cell Res Ther. 2020. PMID: 32122393 Free PMC article.
References
-
- da Silvaz Meirelles L, Chagastelles PC, Nardi N. Mesenchymal stem cells reside in virtually all post natal organs and tissues. J Cell Sci. 2006;119:2201–2213. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

